1. Scope of this document
This document contains the assessment and certification criteria for performing audits at applicant organizations/GMP+ Certified Companies which will result in (re)certification for the GMP+ Feed Certification scheme, Feed Safety Assurance (FSA) module.
2. Normative reference(s)
The following documents, in whole or in part, are normatively referenced in this document and are mandatory to comply with. For dated references, only the edition cited applies. For undated references, the latest edition of the referenced document (including any amendments) applies.
- ISO/IEC 17021-1:2015 Conformity assessment – requirements for bodies providing audit and certification of management systems.
- ISO 22003-1:2022(E) Requirements for bodies providing audit and certification of food safety management systems.
- IAF MD 2:2017 - IAF Mandatory Document for the Transfer of Accredited Certification of Management Systems.
- IAF MD 5:2019 - Determination of audit time of Quality, Environmental, and Occupational Health & Safety Management Systems.
- ISO/IEC 17020:2012 Conformity assessment – requirements for the operation of various type of bodies performing inspection.
- ISO/IEC 17025:2017 General requirements for the competence of testing and calibration laboratories.
- F0.1 Rights and Obligations.
- F0.2 Definition list.
- F0.3 Scopes for certification.
- CR1.0 Acceptation Requirements.
- CR3.0 Assessment and Certification of Feed Responsibility Assurrance.
- GMP+ Feed Safety Assurance Module 2020.
3. Terms and Definitions
For GMP+ definitions see F0.2 Definition List. Throughout this document the terminology “through the Certification Body” is used indicating that all activities performed by Critical-, non-critical locations are conducted under the responsibility/liability of the GMP+ accepted Certification Body.
4. Principles
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Chapter 4 |
5. Process requirements
5.1. Pre-certification activities
5.1.1. Application
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.1.1 |
In addition, relevant details of the applicant organization as mentioned under 9.1.1. B) of the ISO/IEC 17021-1:2015 are:
- Gatekeeper files,
- Multi-site certification,
- Number of employees,
- Number of products.
- An up-to-date group structure of the applicant organization, including ultimate beneficiary ownership and management overview, as well as a statement indicating the applicant organization, its ultimate beneficiary owner’s or its management’s involvement in businesses similar to the applicant organization business, if any to confirm that the applicant organization complies with chapter 4 of the F0.1 Rights and Obligations.
And if applicable:
- Number of analysis;
- Accredited analysis;
- Partly accredited analysis
- Not accredited analysis.
5.1.2. Application review
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.1.2 |
In addition:
- Description of activities and/or processes to be assessed by the Certification Body during an audit.
- The scope of certification must not be misleading.
5.1.3. Certification agreement
Before conducting an initial certification audit/inspection, the Certification Body and the applicant organization must conclude a legally enforceable unique certification agreement and during the validity of a GMP+ certificate/temporary acceptance this legally enforceable unique certification agreement remains in force.
Certification agreement issued by a critical-/non-critical location must comply with the template approved by the Certification Body in question.
The Certification Body must be aware that:
- the certification agreement must always be concluded with the applicable legal entity.
- These agreements must be concluded for the provision and description of the applicable certification activities in accordance with the GMP+ Feed Certification scheme.
- It is not allowed to secure conditions in the certification agreement which are conflicting with GMP+ requirements.
- It is not allowed to determine and impose additional requirements to the applicant organization/GMP+ Certified Company other than specified in the GMP+ Feed Certification scheme, unless specified in the internal procedure of the GMP+ Certified Companies.
The following GMP+ specific requirements must be secured in the certification agreement:
- The applicable scope(s)/module names covering GMP+ certification.
- The minimum obliged audit times per scope(s)/module per type of audit/inspection are as stated in Appendix 2, referring to Appendix 2 is insufficient. It is not permitted to deviate from the minimum obliged audit times by way of invoicing based on re-calculation. If a longer audit time is applicable, then this can be done in consultation with the applicant organization/GMP+ Certified Company. In case of Multi-site certification the minimum obliged audit times as mentioned in Appendix 4 must apply.
- Each multi-site location must be secured with its GMP+ registration number and the applicable minimum obliged audit time per type of audit as stated in Appendix 4, referring to Appendix 4 is insufficient.
- The use of the GMP+ logo in accordance with the F0.1 Rights and Obligations.
- The stipulation (if applicable), that, in case of a determined nonconformity of a permitted level of a contaminant, the GMP+ Certified Company is obliged to submit an EWS notification in accordance with the R1.0 Feed Safety Management Systems Requirements.
- The obligated cooperation of the applicant organization/GMP+ Certified Company with ad-hoc audits (as stated in F0.1 Rights and obligations), witness audits and parallel audit (as stated in Acceptation requirements).
- The forwarding of audit reports/audit checklists to GMP+ International.
- The possibility to terminate the certification agreement before the end of the certification cycle.
- If applicable, the unannounced audit (see article 5.2.1.4.2 of this document)
- The possibility in case of an unsatisfactory appeal procedure that the GMP+ certified company can apply the Dispute procedure.
5.1.4. Audit team/inspection assignment
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.2.2 |
Related to Article 9.2.2.1.2 the additional requirements as stated in article 4.3.6 of the CR1.0 Acceptation requirements apply.
5.1.4.1. Rotation of auditors/inspector
An auditor must not be assigned to the same GMP+ Certified Company more than 3 consecutive years. Should an alternative auditor not be available, an exemption can be made by the Certification Body and the period can be extended for a maximum of 3 consecutive years. The decision must be motivated and documented.
Rotation of auditors/technical expert scope registered laboratory:
- The auditor and technical expert may only perform the desk study of the same GMP+ Certified Company six consecutive times. Then rotation of the auditor and technical expert must take place
Rotation of Inspector:
- A new inspector must be assigned after 3 consecutive inspections.
5.1.5. Audit plan
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.2.3 |
In addition, the applicant organization/GMP+ certified company must provide on request of the certification body the following documentation:
- Organizational chart and short process descriptions,
- List of GMP+ assured products,
- Information about the production site, and / or subcontractors,
- The FSMS Manual on site during the audit (paper or electronic version).
- List of applicable regulations,
- Any other information the auditor/operator may find useful/relevant.
The selection of all relevant personnel to be interviewed must adequately cover every relevant functional area.
For the surveillance or re-certification audit, the GMP+ certified company must provide the certification body with the following documentation/information:
- Changes in organization,
- Changes in FSMS Manual,
- Changes in applicable legislation,
- Scope information,
- And any other information which is relevant.
An audit plan is not applicable for the scope Inland waterway transport and short sea shipping of feed.
5.2. Certification process
5.2.1. Audits/Inspections
5.2.1.1. General
A Certification Body accepted by GMP+ International under the GMP+ Feed Certification scheme is entitled to certify companies through the Certification Body who have an interest for 1 or more GMP+ scopes for the feed sector as specified in GMP+ Feed Certification scheme.
The applicant organization/GMP+ Certified Company must cooperate fully with audits as specified in this document. During the audits/inspections the process for which the company is certified must be operational to be verified. Auditing may include taking samples of products and laboratory testing.
Through the Certification Body, the assessment will take place by means of an audit/inspection at the applicant organization/GMP+ Certified Company for conformity of the applicable scope(s) based on the general criteria as specified in Appendix 1 and the additional assessment criteria in the checklists.
This is applicable for the following audits:
- Initial certification audit (ICA)
- Announced surveillance audit (ASA)
- Unannounced surveillance audit (USA)
- Inspection
- Recertification audit (RCA)
- Expansion audit
- Document assessment (DA)
In addition:
- The assessment for the scope Registered laboratory will be performed by means of a desk study (or on site if applicable).
- The administrative assessment of the scope Laboratory testing, if all analyses are accredited in accordance with ISO/IEC17025 must be performed once per year.
- On site assessment of the scope Laboratory testing if not all analyses are accredited in accordance with ISO/IEC17025 must be performed once per year for the non-ISO/IEC 17025 accredited analyses.
- On site assessment of the scope Laboratory testing if the laboratory is not accredited in accordance with ISO/IEC17025 must be performed once per year for system assessment.
In addition, special audits can also be carried out (see article 5.2.2.).
The certification cycle has a maximum duration of 3 years. All requirements must be assessed during each of the following audits: initial certification-, (un)announced surveillance- and recertification audit.
The minimum obliged audit times and the frequency are determined in Appendix 2 and Appendix 4.
In case a GMP+ Certified Company changes during the certification cycle their activities and/or location the GMP+ certified company must be audited on-site through the Certification Body.
This is applicable for production, transport and storage & transshipment. The GMP+ audit times must apply. It is up to the Certification Body to decide if the initial certification- or surveillance audit must be performed.
5.2.1.2. Opening meeting
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.4.2 |
In addition, not applicable for scope Inland waterway transport and short sea shipping of feed
5.2.1.3. Initial certification audit
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.3.1 |
| ISO 22003-1:2022(E) | Article 9.3.2 up to and including 9.3.4 |
A GMP+ certificate may or may not be granted, depending on whether the assessment criteria of this document are met. An Initial certification audit stage 1 must be conducted within 3 months after concluding a certification agreement with the applicant organization. The interval between stage 1 and stage 2 is maximum 4 months.
When the specific conditions as described in Appendix 5 “Not at the GMP+ Certified Company location” and/or Appendix 6 “Remote Audits” are met, the Initial certification audit can be performed accordingly.
Scope laboratory testing:
The most important analyses must be assessed during the initial certification audit. All analyses must be assessed during the certification cycle.
5.2.1.3.1. Temporary acceptance
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.3.1.2 |
| ISO 22003-1:2022(E) | Article 9.3.2 up to and including 9.3.4 |
It is possible, on the basis of a positive assessment of stage 1 of the feed safety management system documentation, to issue a temporary acceptance (maximum 4 months) as part of an initial certification audit at a company which is starting its GMP+ activities.
Regarding the location of the assessment the following applies:
- When a company carries out production and/or storage and/or transport activities (tractionairs excluded), then part of the assessment of the quality documentation must take place at the company location(s) so that the infrastructural facilities can be assessed.
- If the company carries out other activities, then part of the assessment of the quality documentation may take place at the company location(s) if the Certification Body considers this necessary.
The entire certification process must be finished within the validity of the temporary acceptance including the updating of the GMP+ Company Database (including status and certificate dates) through the Certification Body.
Companies not eligible for a temporary acceptance are:
- Companies transferred from another Certification Body.
- Companies who were GMP+ certified or had a temporary acceptance in the past.
5.2.1.4. Surveillance audits
All requirements must be verified during surveillance audits.
The first surveillance audit must be executed each 12 months, plus and minus two months, after the certification decision date.
The second surveillance audit must be executed each 24 months, plus and minus two months, after the certification decision date.
When the specific conditions as described in Appendix 5 “Not at the GMP+ Certified Company location” and/or Appendix 6 “Remote Audits” are met, the surveillance audit can be performed accordingly.
5.2.1.4.1. Announced surveillance audit
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.6.2 |
5.2.1.4.2. Unannounced surveillance audit
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.6.2 |
Certification Bodies must not schedule the unannounced surveillance audit within 2 months prior to or following the execution of other audits (initial certification, recertification and announced surveillance audits). Every twelve (12) months of the audit cycle, each GMP+ Certified Company can specify 15 days in that year during which the unannounced surveillance audit cannot be performed. If not indicated in advance the unannounced surveillance audit cannot be refused. It is up to the Certification Body to decide whether the legitimate motivation to postpone the unannounced surveillance audit, is justified.
Examples of legitimate postponing of the unannounced surveillance audit are:
- The Certification Body cannot visit the site of the GMP+ Certified Company because its flooded or there are other extreme weather conditions.
- The location of the GMP+ Certified Company is closed (yearly closing, maintenance, holiday) or the location of the GMP+ Certified Company is not conducting GMP+ activities (seasonal work).
The following prior notice periods to perform the unannounced surveillance audit are applicable:
- GMP+ Certified Companies (producers) located in the Netherlands: not allowed.
- GMP+ Certified Companies (producers) located in Germany: one working day.
- GMP+ Certified Companies (producers) located in other countries in Europe: two working days.
- GMP+ Certified Companies (producers) located outside Europe: three working days.
There are several options:
A: Mandatory unannounced surveillance audit
The unannounced surveillance audit is mandatory for GMP+ Certified Companies located in Europe certified for one of the following scopes:
- Production of compound feed (incl. pet food),
- Production of premixtures,
- Production of feed additives,
- Production of feed materials (incl. pet food).
The unannounced surveillance audit will replace one of the announced surveillance audits during the certification cycle and must be registered in the GMP+ Company Database.
Option B: Voluntary unannounced surveillance audit
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.6.2 |
Those who apply for the voluntary unannounced audit, will be obliged to participate during the whole certification cycle. The unannounced surveillance audit will replace one of the announced surveillance audits during the certification cycle and must be registered in the GMP+ Company Database.
B1) For Europe GMP+ Certified Companies certified for the following scope(s) :
- Trade in feed,
- Storage and Transshipment of feed,
- Road transport of feed,
- Rail transport of feed,
- Affreightment (all scopes).
European GMP+ Certified Companies (including GMP+ Certified Companies located in the Netherlands and Germany) who are certified for 1 of the production scopes and therefore obligatory participate in the unannounced surveillance audit for the production scope, can decide whether they want to apply the unannounced surveillance audit also for 1 of the scopes mentioned under option B1.
B2) For all GMP+ Certified Companies outside Europe certified for any GMP+ scope.
The unannounced audit can on a voluntary basis be applied for all scopes in any country.
5.2.1.5. Inspection
| Relevant requirements must apply | |
| ISO/IEC 17020:2012 | Article 7.1 |
An certification inspection will be performed through the Certification Body in order to assess whether the company meets the criteria set out the checklist for Inland waterway and short sea shipping. The certification inspection must be conducted within 3 months after concluding a certification agreement with the applicant organization.
5.2.1.6. Recertification audit
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.6.3 |
A GMP+ certificate may or may not be extended, depending on whether the assessment criteria set out in Annex 1 of this document or in the GMP+ checklist Inland waterway transport and short sea shipping of feed are met. Before the period of validity of the certificate expires, the total certification process must be finished including updating of the GMP+ Company Database (status and data of certificate) through the Certification Body.
If a recertification audit/inspection is not carried out before the expiry of the period of validity of the certificate, then an initial certification audit/inspection must be carried out. The GMP+ Certified Company is in the intervening period not GMP+ certified.
If the specific conditions as described in Appendix 5 “Not at the GMP+ Certified Company location” and/or Appendix 6 “Remote Audits” are met, the recertification audit can be performed accordingly.
5.2.1.7. Expansion audit
If a GMP+ Certified Company wishes to expand the range of his already granted certification with an additional scope(s) and the expansion cannot wait until the next audit, the expansion must be assessed through the Certification Body.
An Expansion Audit (stage 1 and stage 2) must only be focused on activities for which the expansion is applicable.
As a result of positive assessment of the expansion the Certification Body must add an additional scope(s) to:
- a GMP+ certificate
- GMP+ Company Database
- GMP+ certification agreement with the GMP+ Certified Company.
5.2.2. Special audits
The following special audits can be applicable, assessment must be done in accordance with Appendix 1 or the checklist Inland waterway transport and short sea shipping of feed.
5.2.2.1. Stricter supervision Audit (SSA)
If 1 or more Major nonconformities are observed through the Certification Body, the GMP+ Certified Company may be placed under stricter supervision for one audit:
- The cost of this audit is at the expenses of the GMP+ Certified Company.
- This audit is in addition to the normal audit cycle.
- The stricter supervision audit will take place, within a period of 3 months.
- Assessment will be based, but not limited to the established major nonconformity.
- A Major Nonconformity can also be handled administratively based on conformity measures formulated by the GMP+ Certified Company.
If 1 or more Critical nonconformities are observed through the Certification Body, the GMP+ Certified Company must at least be placed under stricter supervision:
- The cost of these audits is at the expenses of the GMP+ Certified Company.
- These audits are in addition to the normal audit cycle.
- The stricter supervision audits will be carried out monthly with a minimum of 3 months and a maximum of 6 months.
- Assessment will be based, but not limited to the established critical nonconformity.
- One stricter supervision audit must be conducted on-site. It is up to the Certification Body to decide if further stricter supervision audits are necessary. This decision must be motivated and documented.
5.2.2.2. Repeat audit (RPA) and Repeat Inspection (RI)
A repeat audit/inspection will be performed under the responsibility of the Certification Body. The reason for a repeat audit/inspection may be an EWS alert, complaints or incidents, or other special circumstances.
In principle the repeat audit/inspection is aimed on these reason(s) but can also be aimed at all requirements of the GMP+ Feed Certification scheme.
- GMP+ International may ask the Certification Body to carry out a repeat audit/inspection on short term in principle in the presence of a GMP+ International auditor/inspector and/or a technical expert.
- The repeat audit/inspection must be carried out by a GMP+ auditor. The involved Certification Body must motivate the choice of the GMP+ auditor/inspector and document its decision.
- The deadline will be assessed per case but ultimately determined by GMP+ International.
- The audit/inspection will be on-site. In addition, administrative checks and a sampling may be carried out.
- The required appointments and communication of the repeat audit/inspection will be made with the GMP+ Certified Company by the Certification Body in consultation with GMP+ International.
- In principle the costs of the repeat audit/inspection will be at the expenses of GMP+ International. However, if it appears that 1 or more Critical or Major nonconformities are observed, the costs will be charged to the GMP+ Certified Company.
5.2.3. Extraordinary events
If the Certification Body and/or Critical Location is confronted with an extraordinary event, GMP+ International must confirm this status. Also when the extraordinary event is specific to a company the Certification body must contact GMP+ International to confirm this status. When confirmed by GMP+ International, the Certification Body is obliged to follow the below guidelines based on the IAF Informative Document for Management of extraordinary events or circumstances affecting, Certification Bodies and GMP+ Certified Companies and which are described as follows:
- The GMP+ Certified Company or business location does not exist because it is destroyed by terrorist acts or acts of war, or is taken over by soldiers or rebels and/or pandemic flooding, earthquake, or other man-made and natural disasters. The Certification Body, Critical/Non-Critical Location is informed by the management of the GMP+ Certified Company or business location or receives the information from another source(s). The Certification Body, Critical/Non-Critical Location is obliged to search for confirmation of the fact from a reliable source. After confirmation, the Certification Body withdraws the GMP+ Certificate and GMP+ International is informed directly in writing, including all the relevant details.
- The GMP+ Certified Company or business location is closed by its head office because the region is not safe. The management of the company of the head office informs the Certification Body, Critical/Non-Critical Location. The Certification Body withdraws the GMP+ Certificate and GMP+ International is informed directly in writing, including all the relevant details.
The GMP+ Certified Company or business location cannot be audited because GMP+ International confirms the extraordinary event, the Certification Body, Critical/Non-Critical Location must follow one of the 2 options:
- If the audit frequency cannot be met and assuming that sufficient evidence was collected to provide confidence that the certified management system of the GMP+ Certified Company is effective, considerations may be given to postpone the surveillance or recertification audit for a period not exceeding 3 months. Otherwise the GMP+ Certificate must be suspended by the Certification Body. During the period of suspension the surveillance or recertification audit must be carried out, otherwise the certificate has to be withdrawn by the Certification Body;
- Perform the audit Full remote audit or a Guided remote audit based on the conditions and requirements of Appendix 6 of this document. When the specific conditions as described in Appendix 6 “Remote Audits” are met, the audits can be performed accordingly.
5.2.4. Identifying and Recording audit and inspection findings
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.4.5 and 9.4.6 |
| ISO/IEC 17020:2012 | Article 7.4 (only applicable for the scope Inland waterway and short sea shipping of feed) |
If the applicant organization/GMP+ Certified Company does not comply with the requirements of the GMP+ Feed Certification scheme, the measures and sanctions as specified in Appendix 1 are applicable.
In addition for the scope Inland waterway transport and short sea shipping of feed the following applies: If a “Not Conform” with a description is observed, a GMP+ certificate cannot be issued. The GMP+ certificate can only be issued if the “Not Conform” with a description is resolved.
If a nonconformity is identified in one of the FSA scopes in combination with an equivalent scheme as mentioned in chapter 3 of TS1.2 Purchase or with FRA, ,this applies for both FSA and FRA scopes.
Multi-Site certification:
If non-conformities are observed at the main office, these non-conformities apply to the whole GMP+ Multi-site organization. If non-conformities are observed at the level of a location, this can influence the location and/or the main office. This is to be assessed through the Certification Body.
Audit findings of the individual multi-sites must be considered indicative of the entire system and correction must be implemented accordingly.
5.2.5. Closing meeting
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.4.7 |
In addition, not applicable for scope Inland waterway transport and short sea shipping of feed
5.2.6. Audit report/checklist
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.4.8 |
For all type of audits/inspections, reporting will take place, in accordance with the model reports stated in Appendix 3.
Within a maximum of eight weeks following the execution of the audit/inspection, the Certification Body will send the GMP+ audit report/checklist to the applicant organization/GMP+ Certified Company.
The Certification Body must provide a written GMP+ audit report for each multi-site location being audited. It is also possible to integrate it into the GMP+ audit report of the main office.
If this is the case an overview must be included in the GMP+ audit report of the main office showing when all the locations / companies were audited. In both cases, a conform or a nonconform GMP+ checklist for each multi-site location must be uploaded in the GMP+ Company Database. Evidence for conform requirements can also be added to the GMP+ audit report/checklist of the main office.
If GMP+ International requests the GMP+ audit report/checklist then the Certification Body will make these available immediately. In the event of a repeat audit/inspection, GMP+ International must receive the GMP+ audit report/checklist within 5 working days.
For all type of audits/inspection (including documentation assessment) the following information must be entered into the GMP+ Company Database and shared with GMP+ International within a maximum of eight weeks following the execution of the audit on site:
- Audit findings/checklist;
- Nonconformities (if applicable);
- Final assessment of the applicant organization/GMP+ Certified Company.
5.2.7. Review
The Certification Body must have a process to conduct an effective review of all GMP+ audit/inspection reports/checklists, including, that:
- the information provided by the audit team is sufficient with respect to the certification requirements and the scope for certification;
- for any type of nonconformities, it has reviewed, accepted and assessed the correction and corrective actions;
- that assessment of the applicant organization/GMP+ Certified Company took place in accordance with the applicable scope(s) based on the general criteria as specified in Appendix 1 and the additional assessment criteria in the checklist.
The conclusion and date of the review by the technical reviewer must be documented.
The technical reviewer must perform the review independent, meaning that the technical reviewer could not have been part of the GMP+ audit team, also not as an observer.
5.2.8. Certification decision
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.5 |
In addition, the assessment criteria of Appendix 1 of this document and/or the criteria of the checklist Inland waterway transport and short sea shipping of feed must be met.
5.2.9. Certificate and Temporary acceptance
5.2.9.1. Certificates
A certificate with a maximum period of validity of 3 years may be issued through the Certification Body, calculated from the date of a positive certification decision. The maximum period of validity for the scope Inland waterway transport and short sea shipping of feed is 2 years, calculated from the date of a positive certification decision. The duration of the GMP+ certificate must not exceed the validity of the certification agreement.
Within eight weeks following the execution of the audit/inspection, the certificate will be send through the Certification Body to the applicant organization/GMP+ Certified Company.
For multi-site location it must be clear were the multi-site location is certified for according F0.3 Scopes for certification. The main office must be certified for the scopes covering all activities of the multi-site locations.
For issuing a certificate the following applies:
- The certified multi-site location can be displayed in an Appendix linked to the certificate of the main location.
- Or an individual certificate can be issued per certified multi-site location, stating the following:
- The activities performed for that specific site / legal entity which are covered by this certification,
- There must be traceability with the main certificate, e.g. code.
Helpful tip
If the GMP+ main office is certified for the scopes production of compound feed and trade in feed and the multi-site locations have a transport scope then the GMP+ main office must also be certified for this scope because the management and control of the feed safety management system of the multi-site construction is centrally controlled at the GMP+ main office.
5.2.9.2. Temporary acceptance
A temporary acceptance with a maximum period of validity of 4 months may be issued through the Certification Body. The duration of the temporary acceptance must not exceed the validity of the certification agreement.
However, if, during the initial certification audit (stage 2), the applicant organization does not appear to comply with the GMP+ requirements for the applicable scope(s) based on the general criteria as specified in Appendix 1 then the temporary acceptance must be withdrawn.
For multi-site location the following applies:
- A temporary acceptance will be issued per multi-site locations or mentioned in an Appendix linked to temporary acceptance of the main location.
- It must be clear where the multi-site location is accepted for according F0.3 Scopes for certification.
5.2.9.3. Certificate and Temporary acceptance templates
The Certification Body must put the following text on the certificate or temporary acceptance:
- Text for certificate Feed Safety Assurance
| Name of the Certification Body: GMP+ International registration number of the Certification Body: Certificate GMP+ FSA logo Name, address, location of the GMP+ Certified Company (Address where GMP+ activities take place) Name and EU number of vessel if applicable GMP+ International registration number of the GMP+ Certified Company FIXED SECTION =name CB= declares that there is justifiable confidence that the GMP+ scope(s) =as mentioned in F0.3 Scope for certification= at the GMP+ Certified Company =name of GMP+ Certified Company= comply with the applicable requirements and conditions of the GMP+ Feed Safety Assurance Module 2020. “ The Feed Management system of the whole multi-site construction is certified and the validity of this certification depends on the validity of the certificate of the main office” FREE SECTION See F0.3 Scope for certification - Optional Specification Registered office of the Certification Body Certificate number Start date and end date of certificate |
- Text for temporary acceptance
| Name of the Certification Body: GMP+ International registration number of the Certification Body: Temporary Acceptance Name, address, location of the temporary accepted company (Address where GMP+ activities take place) Name and EU number of vessel if applicable GMP+ International registration number of the temporary accepted company FIXED SECTION =name CB= declares that there is justifiable confidence that the GMP+ scope(s) =as mentioned in F0.3 Scope for certification= at the GMP+ temporary accepted company =name of GMP+ temporary accepted company= comply with the criteria of a stage 1 assessment of the applicable requirements and conditions of the GMP+ Feed Safety Assurance Module 2020. “ The Feed Management system of the whole multi-site construction is temporary accepted and the validity of this temporary acceptance depends on the validity of the temporary acceptance of the main office” FREE SECTION See F0.3 Scope for certification - Optional Specification Registered office of the Certification Body Temporary acceptance number Start date and end date of temporary acceptance |
- Text for a certificate for the scope Registered laboratory:
Name of the Certification Body
GMP+ International registration number of the Certification Body
Certificate
GMP+ FSA logo
TS 4.2 Registered laboratory
Name, location of the GMP+ Certified Company
GMP+ International registration number of the GMP+ Certified Company
The Certification Body =name of the Certification Body= states that GMP+ Certified Company =name GMP+ Certified Company= was audited in accordance with the applicable requirements of the TS 4.2 Registered laboratory and GMP+ Feed Safety Assurance Module 2020.
The Certification Body =name of the Certification Body= states, based on a desk study, that the performance criteria as mentioned in the TS 4.2 Registered laboratory are met for the following analyses:
Operation
Material/matrix
Feed materials
Feed additives and premixtures
Feed (compound feed and complementary feed)
Mycotoxins
Aflatoxin B1
Not Applicable
Dioxins/PCBs
Sum of dioxins and dioxin-like PCBs
Dioxins
Dioxin-like PCBs
Non-dioxin-like PCBs
Heavy metals
Arsenic
Lead
Cadmium
Mercury
Not Applicable
Fluorine
Not Applicable
Pesticides
Pesticides
Registered office of the Certification Body
Certificate number
Start date and end date of certificate
In addition the following applies for all certificate templates and temporary acceptance:
- The data of the GMP+ Certified Company/temporary accepted company must exactly be the same as registered in the legal business registration.
(for example Chamber of Commerce/registration at competent authority, tax/vat number) - It is mandatory to show the GMP+ FSA logo on the certificate.
- It is not permitted to use the GMP+ FSA logo on a temporary acceptance. In addition, the document may not be called a “certificate” but must be designated as a “temporary acceptance”.
- It is not permitted to use the logos of Critical Location and non-Critical Location on the GMP+ certificate and temporary acceptance other than the GMP+ accepted Certification Body.
- The start date of the certificate/temporary acceptance is a date which is in any event equal or after the date of the positive certification/temporary acceptance decision.
- In case of expansion of scopes the end date of the valid GMP+ certificate may not be extended. The Certification Body can also grant the GMP+ Certified Company a new GMP+ certificate for the additional scope.
- It is not permitted to specify brand names in any way whatsoever on the certificate or temporary acceptance.
- (Contractual) conditions/ requirements are not allowed on the GMP+ certificate/temporary acceptance
5.3. Suspension or Withdrawal of a certificate and Temporary acceptance
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.6.5.1 |
If it is established that a GMP+ Certified Company/temporary accepted company no longer complies with the requirements, sanctions must be imposed immediately, through the Certification Body, in accordance with Appendix 1.
The auditor must report Critical non-conformities as specified in Appendix 1 immediately to the responsible GMP+ coordinator.
In deviation, for the scope Inland waterway transport and short sea shipping of feed the following applies: If a “Not Conform” with a description is observed, the GMP+ certificate must be withdrawn. A GMP+ certificate can only be issued if the “Not Conform” with a description is resolved.
The responsible GMP+ coordinator must inform GMP+ International within 2 working days of non-compliance with the requirements by using the form Audit Finding Notification Critical Non-conformity in case of:
- A critical non-conformity,
- Suspension of the GMP+ certificate ,
- Withdrawal of the GMP+ certificate.
The GMP+ Company database must be adapted through the Certification Body to status: “suspended or withdrawn” with reason: “does not meet the requirements” within 2 working days. When the Certification Body has determined a critical non-conformity it is not allowed to withdraw the GMP+ Certificate with the reason of withdrawal “cancellation of agreement”. Once the certificate has been suspended or withdrawn the company cannot participate in the GMP+ Feed Certification scheme under any Gatekeeper Protocol.
GMP+ International is entitled to publish the suspended/withdrawn certificates.
5.4. Transfer to another Certification Body
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 9.5.3.3 |
During the validity of a GMP+ certificate, a GMP+ Certified Company is entitled to transfer to another Certification Body. Such transfer is subject to the following conditions:
5.4.1. Pre-transfer review
The departing Certification Body is obliged to make available all relevant information/data to the accepting Certification Body/Critical Location in question.
The accepting certification body must have a process for obtaining sufficient information in order to take a decision on certification and inform the transferring GMP+ certified company of the process. This information must as a minimum include arrangements regarding the certification cycle.
The accepting certification body must determine the competence criteria for personnel involved in pre-transfer review. The review may be conducted by one or more persons. The individual or group conducting the pre-transfer review must have the same competence that is required for an audit team appropriate for the scope of certification being reviewed.
The accepting Certification Body/Critical Location must carry out a review of the certification of the GMP+ Certified Company. This review must cover the following aspect and its findings must be documented:
- confirmation that the GMP+ certified company’s certification falls within the accepted scope of the departing and accepting certification body;
- the reasons for seeking a transfer;
- that the site or sites wishing to transfer certification hold a valid certificate;
- the initial certification or most recent recertification audit reports, and the latest surveillance report; the status of all outstanding non-conformities that may arise from them and any other available, relevant documentation regarding the certification process.
- for the scope Inland waterway transport and short sea shipping of feed an evaluation of the last checklist to established if a “Non Conform” with description were observed. This evaluation can include other relevant documentation, regarding the (re)-certification process i.e. notes, etc.
- if one outstanding non-conformity has the classification Critical transfer is not allowed;
- complaints received and action taken;
- any current engagement by the transferring GMP+ certified company with regulatory bodies relevant to the scope of the certification in respect of legal compliance;
- Confirmation that the GMP+ Certified Company has no unfulfilled contractual obligations with the departing Certification Body.
5.4.2. Certification process during transfer
After successful pre-transfer review the following conditions apply:
- The accepting Certification Body, Critical/Non-Critical Location has to conclude a GMP+ Certification Agreement with the applicant organization (see article 5.1.3.) before submitting the business request “Change of Certification Body” to GMP+ International. A new certification cycle must be started. An Initial certification audit must be carried out.
- Open non-conformities issued by the departing Certification Body should be closed before transfer, otherwise the non-conformities must be closed by the accepting Certification Body/Critical Location during the Initial certification audit for the scope Inland waterway transport and short sea shipping of feed an open “Non Conform” with description established during the last inspection must be closed by the accepting Certification Body/Critical location during the initial inspection.
- A new certificate must be issued within 5 months of the date of transfer. It is not allowed to transfer a GMP+ Certificate from the departing Certification Body to the accepting Certification Body. A Certification Body is not allowed to accept transfer of a Company which GMP+ Certificate has been suspended or withdrawn. Except for withdrawn on “cancellation of agreement”.
5.4.3. Cooperation between the departing and accepting Certification Bodies
| Relevant requirements must apply | |
| IAF Mandatory Document for the Transfer of Accredited Certification of Management Systems – IAF MD 2:2017 | Article 2.4 |
6. Exclusion of GMP+ International liability
GMP+ International has no liability whatsoever with respect to the assessment of applicant organizations/GMP+ Certified Companies through the Certification Bodies. The Certification Bodies in question will indemnify GMP+ International in this respect.
7. Tariffs
The Certification Body will use its own tariff. On behalf of GMP+ International, through the Certification Body, relevant tariff as listed in GMP+ CR4.0 Tariffs are charged.
8. Disputes between Certification Bodies and GMP+ certified companies
Disputes between Certification Bodies and applicant organization/GMP+ Certified Companies with respect to the assessment will initially be handled in accordance with the dispute regulation of the Certification Body. If this does not lead to a solution then the dispute can be handled in accordance with the F0.5 Disputes procedure.
Appendix 1: Assessment criteria and Sanctions for audits GMP+ FSA
Non-conformities are to be classified based on:
- The general assessment criteria as mentioned in this Appendix
- The specific assessment criteria as shown in the checklists.
The sanctions specified must be imposed as a minimum. Through the Certification Body it is allowed to impose stricter sanctions.
If in this table is mentioned certificate it also applies for the temporary acceptance.
| Classification: Minor non-conformity | ||||
| Description | Consequences | Period to close | ||
| | ICA/RCA | SA | ||
| GMP+ Certified Companies:
| < 10 non-conformities | Certificate can be issued | Certification can be continued | during next on-site audit |
| ≥ 10 non-conformities | Certificate cannot be issued | Certification can be continued | within 6 weeks | |
| Classification: Major non-conformity | |||
| Description | Consequences | Period to close | |
| ICA/RCA | SA | ||
| GMP+ Certified Companies:
| Certificate cannot be issued | Certification can be continued but a stricter supervision audit may be performed (see Art. 5.2.2.1) | within 6 weeks |
| Classification: Critical nonconformity | |||
| Description | Consequences | Period to close | |
| ICA/RCA | SA | ||
| GMP+ Certified Companies:
| Certificate cannot be issued | *Level 1. Certification can be continued but stricter supervision audits must be performed (see Art. 5.2.2.1) | Within 2 weeks |
| *Level 2. Certificate must be suspended: maximum 3 months | |||
| Lifting of *level 2: Certificate can be continued only possible if the Certification Body can close the critical non-conformity during stricter supervision audit (see Art. 5.2.2.1) | |||
| *Level 3. Certificate must be withdrawn at least 1 year excluded from participation in the GMP+ Feed Certification scheme, as well as all Gatekeeper Options | |||
| GMP+ Certified Companies:
| Certificate cannot be issued | *Level 1. Certificate must be suspended: maximum 3 months | |
| Lifting of *level 1: Certificate can be continued only possible if the Certification Body can close the critical non-conformity during stricter supervision audit (see Art. 5.2.2.1) | |||
| *Level 2. Certificate must be withdrawn at least 1 year excluded from participation in the GMP+ Feed Certification scheme, as well as all Gatekeeper Options | |||
* Sanctions can be applied starting at any level.
Appendix 2: Frequency and Audit times
Frequency
Audits must be carried out in accordance with the following cycle.
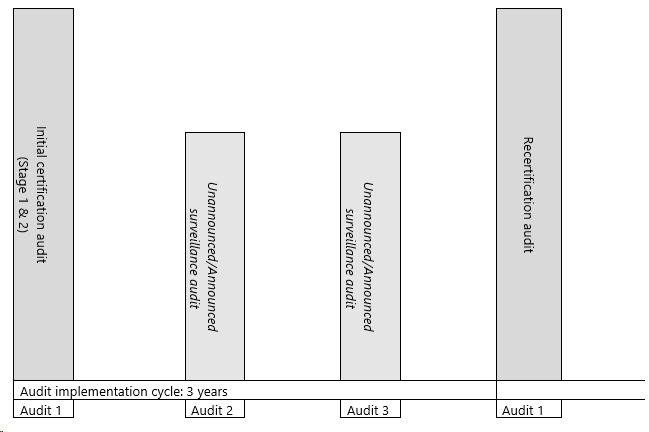
This is a qualitative representation of the audit cycle for executing GMP+ audits.
The audit times are expressed in days, one day is eight hours. Audit times includes stage 1 & 2 for initial certification audit. The tables in this Appendix provide mandatory minimum audit times including preparation and reporting of the audit. The audit time on site must at least be 70% of the minimum obliged audit time for all types of audits (excluded the cases of not on-site Stage 1 audits). When properly documented and justified a reduction to the minimum obliged audit times can be issued to a less complex organization measured by a simple production process, size of the organization, product volume (including a limited number of products), seasonally being active, etc. The GMP+ Certified Company must receive an adapted offer/certification agreement. GMP+ International will check the reasoning and assess this during the annual Certification Body audit. The audit time reduction must be processed in the GMP+ company database.
The Certification Body cannot issue audit time reduction if:
- It exceeds more than 30% of the minimum obliged audit time.
- During the validity of the GMP+ certificate an audit time reduction already exists and no changes in activities have occurred.
- During the last 3 audits at the GMP+ Certified Company 1 Critical non-conformity was established.
- During the last 3 audits at the GMP+ Certified Company 1 Major non-conformity was established with a structural character or the Major non-conformity resulted in feed safety hazard.
- During the last 3 audits at the GMP+ Certified Company 20 or more Minor non-conformities were established.
- Audit time is reduced for a combined audit.
- Table 2 of this appendix is applied.
In addition, audit time reduction cannot be issued on the audit times of Appendix 4 of this document.
Through the Certification Body audit time reduction can only be granted on the initial certification audit if the Certification Body can demonstrate that they certified the company for another scheme as mentioned in this Appendix and/or an equivalent scheme as mentioned in TS1.2 Purchase and properly documented and justified. Audit time reductions are not allowed to be used for re-calculation of the minimum obliged audit times, except when during the initial certification audit as stated above.
This temporary deviation from the audit time is valid as long as:
- no changes take place in the activities and organisation of the GMP+ Certified Company
- no changes are made to this Appendix regarding audit times.
- The GMP+ Certified Company does not transfer to another Certification Body. If the GMP+ Certified Company transfers to a new Certification Body, the new Certification Body has to assess if an audit time reduction can be issued.
In the event of repeat audits and stricter supervision audits as specified in article 5.2.2, the time will apply which is considered necessary through the Certification Body or GMP+ International. The audit times may increase if EWS, complaint, exemptions, incidents, etc, have to be investigated through the Certification Body.
The ranking must be applied as follows:
- Production of compound feed
- Production of premixtures
- Production of feed additives
- Production of feed materials
- Production of Pet food
- Trade in feed
- Storage and Transshipment of Feed
- Transport of feed
- Affreightment
For the calculation of the minimum obliged (Initial Certification audit (ICA); (Un)Announced Surveillance audit (USA/ASA) and Recertification audit (RCA)) audit time for a single site the following formula will be used:
Ts=TD+ TH1 (if applicable)+TFTE
Where:
Ts: minimum audit time
TD: is the basic audit time, in days;
TH1: is the number of audit days for additional GMP+ scopes;
TFTE: Is the number of audit days per number of employees;
| Table 1 | |||||
| Minimum obliged audit times1 : Ts=TD+TH1 (if applicable)+TFTE | |||||
| | Basic audit times in days | N° audit days for each additional GMP+ scope | Total no. of employees (FTE7 relevant for personnel related to all GMP+ activities, expressed in audit days ) | Deductible GMP+ audit times in case of a combined audit with a valid version of equivalent schemes/scopes as mentioned in TS1.2 Purchase | Deductible GMP+ audit times in case of a combined audit5 |
| GMP+ scopes | TD | TH1 | TFTE | | |
| Production of compound feed2+3+6 | 1,75 | 0,1875 | 1 to 19 = 0 | Reduction of maximum 75% of the minimum obliged audit times. | Reduction of maximum 50 % of the minimum obliged audit times. |
| Production of premixtures6 | 1,75 | 0,1875 | |||
| Production of feed additives6 | 1,75 | 0,1875 | |||
| Production of feed materials3+ 6 | 1,125 | 0,1875 | |||
| Trade in feed3+8+9 | 1,00 | 0,1875 | |||
| Storage and Transshipment of feed8+9 | 1,00 | 0,1875 | |||
| Transport of feed4+8+9 | 1,00 | 0,1875 | |||
| Affreightment8+9 | 0,70 | N.A. | |||
| 1 Applicable for all type of audits (special audits according to Article 5.2.2 excluded). 2 Without the use of critical feed additives and/or veterinary medicinal product the audit times may be reduced with a maximum of 0,25 days per site. 3 Applicable for pet food. 4 For road transport the affreightment of road transport is included. 5 ISO9001 and/or ISO22000 and/or IFS food and/or BRC production and/or FSSC 22000. 6 When an organization deploys workers in shifts and the products and/or processes are similar, the FTE number will be calculated based on employees on the main shift (including seasonal workers) plus office workers. 7 Number of employees is including part-time workers calculated as percentage of FTE. 8 If an invoicing address is applicable the minimum obliged audit time is 0,125 days. 9 If a PO-box is applicable the minimum obliged audit time is 0,125 days. | |||||
| Table 2 | |||||||||||
| Additional requirements for audit time calculation | |||||||||||
| Each additional production location1 audited | 1 day per type of audit. | ||||||||||
| Each additional production location1 audited, producing compound feed with the use of critical feed additives and/or critical veterinary medical product | 1,25 day per type of audit. | ||||||||||
| Trade in feed ≤ 2 TFTE ² | Reduction of maximum 0,1875 day per type of audit. | ||||||||||
| Trade in feed, “forage trade”, ≤ 5 products | Reduction of maximum 0,5 day per type of audit. | ||||||||||
| Trade to livestock farms | Reduction of maximum 0,75 day per type of audit. | ||||||||||
| Storage and Transshipment of feed ≤ 5 TFTE ² | Reduction of maximum 0,1875 day per type of audit. | ||||||||||
| Road transport of feed, ≤ 2 TFTE ² | Reduction of maximum 0,63 day per type of audit. | ||||||||||
| Road transport of feed, 3 - 5 TFTE ² | Reduction of maximum 0,40 day per type of audit. | ||||||||||
| Rail transport of feed | Reduction of maximum 0,30 day per type of audit. | ||||||||||
| Antibiotic-free production site (always additional) | 0,25 day per type of audit | ||||||||||
| Dioxin-monitoring in feed for laying hens (always additional) | 0,125 day per type of audit | ||||||||||
| QM-Milch³ (always additional) | 0,125 day per type of audit | ||||||||||
| 1 Requirements for an additional production site : A location who has a legal or contractual link with the GMP+ certified main office of the organization and be subject to a common management system, which is laid down, established and subject to continuous surveillance and internal audits by the main office. This means that the main office has rights to require that the locations implement corrective actions when needed in any location. Where applicable this must be set out in a formal agreement between the central office and the locations. For each additional FSA scope 0,1875 must be added. ² Only applicable if the company is certified for a single scope. If the company is certified for multiple scopes the total number of FTE involved in GMP+ activities is applicable. See example in helpful tip. ³ In addition, a deviation of the minimum obliged audit times, including Initial Certification Audit (ICA), can be applicable if the following requirements are met:
| |||||||||||
Helpful tip
Example: A trader with 1 FTE and the additional scope Storage and Transshipment of feed with 2 FTE’s results in 3 TFTE related to GMP+ activities. As a result, the trader in not fitting the criteria Trade in feed ≤ 2 TFTE.
| Table 3 | |||||
| GMP+ FSA module | Number of analyses | Audit/ Inspection frequency | Minimum audit/inspection times in days, audit time reduction is not allowed, unless one of the footnotes is applicable |
| |
|
|
|
| Initial or re-certification audit/inspection | Announced/unannounced surveillance audit | Comment |
| Scope: Laboratory testing |
|
|
|
| 1 + 2 + 3
|
| ISO/IEC17025 accredited | < 5 | 1x / year | 0,25 | 0,25 | |
| 5-15 | 1x / year | 0,38 | 0,38 | ||
| >15 | 1x / year | 0,50 | 0,50 | ||
| Partially ISO/IEC17025 accredited | < 5 | 1x / year | 0,69 | 0,69 | |
| 5-15 | 1x / year | 1,00 | 0,88 | ||
| >15 | 1x / year | 1,19 | 1,19 | ||
| Not ISO/IEC17025 accredited |
| ||||
| Main location (incl. system) | < 5 | 1x / year | 1,00 + 1,00 | 0,81 + 0,81 | |
| 5-20 | 1x / year | 1,19 + 1,19 | 1,19 + 1,19 | ||
| >20 | 1x / year | 1,50 + 1,50 | 1,19 + 1,19 | ||
| Secondary location (analyses) | < 5 | 1x / year | 0,63 | 0,69 | |
| 6-20 | 1x / year | 0,81 | 0,94 | ||
| >20 | 1x / year | 1,00 | 1,19 | ||
| Scope: Registered laboratory |
|
|
|
|
|
| outsourcing all analysis |
| 1x / year | 0,50 | 0,50 | 4 |
| partly outsourcing analysis |
| 1x / year | 1,006 | 1,006 | 4 +5 |
| without outsourcing of any analysis |
| 1x / year | 1,006 | 1,006 | 5 |
| Scope: Inland waterway transport and short sea shipping of feed |
| 1x / 2 years | 0,25 | N.A. |
|
| 1 Types of laboratories: - If the laboratory is accredited for more than 50 analyses according to ISO/IEC17025 the minimum audit time may be raised with 0,094 days. - Where the laboratory is not accredited according to ISO 17025; both the material specialist and the auditor visit for system assessment. 2 If a laboratory is certified for both TS4.1 Laboratory testing and ISO 9001; 2000 or ISO22000 then an audit time reduction of 35% may be applied under the condition that the laboratory has the applicable ISO certificate(s). The reduced audit times may only be used if all secondary locations are working under the quality system of the main location. The system requirements and analyses will be assessed at the main location. At the secondary locations only, the analyses are assessed. 3 The requirements of the scope Laboratory testing and the other GMP+ scopes are so different that a combined audit for the scope Laboratory testing and one or more of the other GMP+ scopes will not give any audit time reduction. 4 The audit times are for the assessment of one analysis. For the assessment of each outsourced additional analysis 0,5 hours must be added. 5 The audit times are for the assessment of one analysis. For the assessment of each performing additional analysis 2,0 hours must be added. 6 The audit times for auditing TS4.2 Registered Laboratories may be reduced up to 50% if the assessment will be performed in combination with the scope Laboratory testing. The audit times auditing TS4.2 Registered Laboratories may be reduced up to 50% if the company has an accreditation in accordance with ISO/IEC17025. | |||||
| Additional audit times in days for assessing Gatekeeper files | |||
| No. Gatekeeper files | minimum of | TS1.2: 4.3.4 Purchase of former foodstuffs 4.3.6 Purchase of feed materials of mineral origin 4.3.7 Purchase of processed feed materials | TS1.2: 4.3.2 Purchase of unprocessed grains, (oil)seeds and legumes out of a collect chain 4.3.5 Purchase of palm oil 4.3.8 Purchase of feed for feed trial 4.4.1 Purchase of road transport 4.4.2 Purchase of inland waterway transport 4.4.3 Purchase of storage and transshipment 4.5.2 Raw material for soap stock splitting |
| 1 to 5 | All | 0,125 per file | 0,063 per file |
| 6 to 10 | 5 | 0,125 per file | 0,063 per file |
| 11 to 15 | 6 | 0,125 per file | 0,063 per file |
| 16 to 30 | 7 | 0,125 per file | 0,063 per file |
| 31 to 50 | 8 | 0,125 per file | 0,063 per file |
| 51 to 100 | 9 | 0,125 per file | 0,063 per file |
| > 100 | 10 | 0,125 per file | 0,063 per file |
Appendix 3: Reporting Model or Audit report and Inspection checklist
Reporting Model A:
1 General details
Details of main location:
Name of the GMP+ Certified Company:
Address:
Postal code and location:
Telephone:
E-mail:
GMP+ registration number:
Legal business registration number:
Contact person:
Spoken with:
| Name | Function |
| | |
| | |
Assessment is performed in accordance with GMP+ Feed Certification scheme 2020.
Overview of all business locations (incl. head office) and GMP+ scopes
| GMP+ registration number | Name location | Address Postal code, Place, Country | Applicable GMP+ scopes | Expiry date of current certificate or temporary acceptance: |
| | | | | |
| | | | | |
List of locations in the event of multi-site certification (if applicable)
| GMP+ registration number location | Name of location | Address Postal code, , Place, Country | Applicable GMP+ scopes | Audit date |
| | | | | |
Audit details:
□ Initial certification audit – on si te
□ Initial certification audit – Full remote
□ Initial certification audit – Guided remote audit
□ Announced surveillance audit – on site
□ Announced surveillance audit – Full remote
□ Announced surveillance audit – Guided remote audit
□ Unannounced surveillance audit – on site
□ Unannounced surveillance audit – Full remote
□ Unannounced surveillance audit – Guided remote audit
□ Recertification audit – on site
□ Recertification audit – Full remote
□ Recertification audit – Guided remote audit
□ Expansion audit – on site
□ Expansion audit – Full remote
□ Expansion audit – Guided remote audit
□ Repeat audit
□ Stricter supervision
□ Documents assessment (in the event of a temporary acceptance)
□ Audit/Inspection* time (in days)
□ Combination audit yes/no applicable quality scheme:
□ Other;
Date of document assessment :
Date of audit :
Report date :
GMP+ Certified Companies representative including name and position:
Documents assessed :
Certification Body :
(Lead) Auditor(s) :
Co-auditor 1 :
Co-auditor 2 :
Technical expert(s) :
Name: ________________ Signature (Auditor) ______________________
2 Scope GMP+ Certified Company/locations.
Specify the type of GMP+ Certified Company and its activities. Describe the products and quantities. Specify the nature and the numbers of personnel (permanent, temporary) per location. Describe the organisational structure. Also take note of other companies on the same site or under the same holding (with similar names or incompatible activities). Provide a brief summary of the whole process and documentation of the management system, for example purchasing, production process, storage, sales and transport of main and subsidiary product streams (focusing on the relationship with the activities covered by the application). Also indicate whether the GMP+ Certified Company applies the Gatekeeper principle and describe the activities .
3 Audit objectives.
The audit objectives must describe what is to be accomplished by the audit and must include the following topics:
- Determination of the conformity of the client’s feed safety management system, or parts of it, with audit criteria,
- Evaluation of the ability of the Quality Management System to ensure the GMP+ Certified Company’s organisation meets applicable statutory, regulatory and contractual requirements.
- Evaluation of the effectiveness of the Quality Management System to ensure the GMP+ Certified Company’s organisation is continually meeting its specified objectives.
- as applicable, identification of areas for potential improvement of the management system.
4 Deviation from the audit plan/significant issues impacting the audit program.
Reason for deviation to be mentioned and significant issues impacting the audit program.
5 Which topics have been assessed and concluded.
In general, it must be clear in the report what has been assessed and what was the conclusion of the auditor.
Verification of effectiveness of taken corrective actions regarding previously identified nonconformities, if applicable.
Per audit objective a conclusion must be given.
6 Summary of the assessment and a general conclusion.
Start with a standard phrase such as “The GMP+ Certified Company was audited for a surveillance audit of the GMP+ requirements. The GMP+ Certified Company was assessed for the requirements of the applicable GMP+ scopes”.
Indicate whether the nonconformities observed in the previous audit have been resolved.
Make a summary per location and in total.
Give a brief summary of the general impression of the quality system of the GMP+ Certified Company.
Possible postscript after a final assessment by the technical reviewer: review of additional documents and follow-up.
| Number of audit nonconformities observed | |||||||||
| Location | During previous audit | During audit | At final assessment | ||||||
| | Number of audit nonconformities | Number of audit nonconformities | Number of audit nonconformities | ||||||
| | Critical | Major | Minor | Critical | Major | Minor | Critical | Major | Minor |
| | | | | | | | | | |
Audit conclusion: the GMP+ Certified Company meets/fails to meet requirements of the GMP+ module.
Measures and sanctions: conformity audit, repeat audit, stricter supervision (including period of time), suspension, withdrawal.
7 Appendices
Checklists used, report forms for audit nonconformities.
Note: non-conformities observed must also be recorded in the English/German or Dutch language.
Reporting Model B:
Audit Report/Inspection Checklist*
(This is an impression of the Audit Report/Inspection Checklist*, consult for the latest version always the Audit Report/Inspection Checklist* processed in the GMP+ Database/Audit app)
| Certification Body | |
| Certification Body | |
| Company Details | |||
| GMP+ Registration Number | | ||
| Company Name | | ||
| Company Relation | | ||
| Address | | ||
| Postal Address | | ||
| Legal Business Registration Number | | ||
| Telephone 24/7 | | ||
| Email Address | | ||
| Spoken with, name and function | | ||
| Gatekeeper files | | Number of gatekeeper files - TS1.2 4.3.3 Purchase of feed additives, foodstuffs, pharma products, herbs and spices 4.3.4 Purchase of former foodstuffs 4.3.6 Purchase of feed materials of mineral origin 4.3.7 Purchase of processed feed materials 4.5.1 Other Products and Services | |
| | | Number of gatekeeper files - TS1.2 4.3.2 Purchase of unprocessed grains, (oil)seeds and legumes out of a collect chain 4.3.5 Purchase of palm oil 4.3.8 Purchase of feed for feed trial 4.4.1 Purchase of road transport 4.4.2 Purchase of inland waterway transport 4.4.3 Purchase of storage and transshipment 4.5.2 Raw material for soap stock splitting | |
| Number of FTE’s | | ||
| Vessel Name | | ||
| Vessel Owner | | ||
| Vessel Registration Number/EU Number | | ||
| Vessel Size in Tons | | ||
| Total Cubic Content | | ||
| Number of Holds | | ||
| Type of Hatch Cover | | ||
| Floor Type (steel, wood) | | ||
| Certification | ||||
| Scope | Module | Certified Since | Start Date | End Date |
| | | | | |
| Company Relation | |
| Connected To | Company Relation |
| Audit/Inspection* Details | |
| Audit/InspectionDate | |
| Report Date | |
| Certification Body | |
| Certification Body - GMP+ Registration Number | |
| (Lead) Auditor/Inspector | |
| Guide | |
| Co-Auditor 1 | |
| Co-auditor 2 | |
| Reviewer | |
| Observer | |
| Technical/Material Expert | |
| Remote | Yes/No Method |
| Audit/Inspection* Type | |
| Audit/Inspection* times (in days) | |
| Combined Audit | |
| Certificate Combined Scheme | Yes/No Validity |
* Initial Certification audit (ICA) Surveillance audit (SA) Unannounced Surveillance audit (USA) Recertification audit (RCA), Compliance audit (CA), Stricter Supervision audit (SSA), Repeat audit (RPA), Document assessment (DA)
| Scopes and Modules of the audit | |
| | |
Audit Objectives
| The audit objectives must describe what it is to be accomplished by the audit and must include the following topics: a) Determination of the conformity of the client’s feed safety management system, or parts of it, with audit criteria, b) Evaluation of the ability of the Quality Management System to ensure the GMP+ Certified Company’s organisation meets applicable statutory, regulatory and contractual requirements, c) evaluation of the effectiveness of the Quality Management System to ensure the GMP+ Certified Company’s organisation is continually meeting its specified objectives, d) As applicable, identification of areas for potential improvement of the management system. |
Deviation from the audit plan/significant issues impacting the audit program.
| Reason for deviation to be mentioned and significant issues impacting the audit program. |
General Information
| The defined processes and documentation of the management system developed by the GMP+ certified company/applicant organization. |
GMP+ Certified Company/Location
| The defined processes and documentation of the management system developed by the GMP+ certified company/applicant organization. |
| Audit Requirements | ||||
| Art. No | Scope/Activity | Modules | Audit Topic | Compliance |
Verification of effectiveness of taken corrective actions regarding previously identified nonconformities, if applicable.
Audit Conclusion
| Per audit objective an conclusion must be given. | |
| | |
| Final Assessment | |
| Approved/Not approved | |
| Date, place | Signature Auditor, |
| Date, place | Signature Reviewer, |
| Date, place | Signature Client, |
Appendix to the report NCR form yes/no
* When the terminology inspector / inspection / inspection checklist is used this refers to the scope Inland waterway transport and short sea shipping of feed as secured in CR 3.0.
Appendix 4A: Multi-site certification
Multi-site certification is possible:
- at a GMP+ Certified Company with a main office with 100% subsidiaries, or
- at a group of companies which have joined together as a quality community.
A multi-site organization does not have to be a unique legal entity, but all multi-site locations must have a legal or contractual link with the main office of the multi-site organization and be subject to a common management system, which is laid down, established and subject to continuous announced surveillance and internal audits by the main office. This means that the main office has rights to require that the multi-site locations implement corrective actions when needed in any multi-site location. Where applicable this must be set out in a formal agreement between the main office and the multi-site locations.
Multi-site certification is not to be used if various independent companies have joined together in a branch organisation, union, federation, association, via an independent consultancy office or similar.
Multi-site certification is not permitted for the FSA scopes:
- Production of Compound Feed;
- Production of Premixtures;
- Production of Feed Materials;
- Production of Feed Additives.
- Laboratory testing;
- Registered laboratory;
- Inland waterway transport and short sea shipping of feed.
Multi-site certification is permitted for all FSA scopes of:
- Trade in feed;
- Storage and Transshipment of feed
- Transport of feed;
- Affreightment.
Helpful tip
If, for example, a group of companies consist of multiple production locations and storage locations, the production locations in this group cannot be certified under multi-site but for the storage locations this is possible.
1. General requirements
- The multi-site organization falls under the same quality system which is managed by the main office. This quality system complies with the relevant GMP+ scope(s) and there must be compliance at all multi-site locations with the relevant GMP+ requirements (see also the helpful tip under Certification).
- The same methods and procedures are used at the multi-site organization.
- Corrective actions may be imposed from the main office on multi-site locations.
- There must be a written agreement between the multi-site locations the main office. This agreement must be signed by all the participating parties and the signed agreement must be present at the main office and available to the auditor. The agreement will include at least:
- a commitment by the multi-location to the main office that it will comply with the requirements set in the quality system.
- that corrective actions imposed by the main office are binding
- that the above applies to all feed activities (and therefore those which are carried out more or less independently).
- All the multi-site locations are included in the programme of internal audits. The internal audit must be performed 1 x per year at all multi-site locations.
- The main office must show that it is able to collect data from every multi-site location, to analyse the data and, where necessary, to implement changes with respect to:
- System documents and changes
- Management review
- Complaints handling
- Corrective actions
- Planning of internal audits and improvement measures.
- In case of unprocessed products all multi-sites locations must be located in the same country or in the bordering regions of neighbouring countries.
1.1 Certification
Before an initial certification audit can take place, a unique certification agreement/certification agreement template including the main office and the multi-site locations must be concluded and also the internal audit report must be available to be handed over to the Certification Body for assessment.
Helpful tip
If the main office is certified for a production scope and the multi-site locations are certified for a transport scope and/or a trading scope, the main office must also be certified for this scope (transport and/or trade) because the management and control of the feed safety management system lies centrally at the main office.
Audit frequency for a multi-site organization:
- With a main office and equal or less than 20 multi-site locations, all multi-site locations must be audited at least once during one certification cycle.
- With a main office and more than 20 multi-site locations, all multi-site locations must be audited at least once during two consecutive certification cycles.
The main office must be audited on-site annually.
If a new multi-site location joins a multi-site organization not during a regular audit, an assessment of the relevant subjects must take place at the main office, this can be done remotely. The new multi-site location must be audited in the current certification cycle. When requirements are met, Appendix 5 and/or Appendix 6 are met, these appendices can be applied.
Minimum obliged audit times in days per for multi-site organizations
| Location | Number of FSA employees*/ products | Minimum time expenditure per FSA audit per location |
| Main office | Audit times as mentioned in Appendix 2 increased with extra audit times per included multi-site location of 0,251+3 day up to a maximum of 1,25 day. | |
| Multi-site location Trade in feed | ≤ 5 products 6-15 products >15 products | 0,25 0,375 0,50 |
| Multi-site location Storage and Transshipment of feed | | 0,25 |
| Multi-site location Road transport of feed. | ≤ 5 FTE 2 6-15 FTE 2 >15 FTE 2 | 0,25 0,375 0,50 |
| Multi-site location Road transport of feed, tractionair | | 0,125 |
| Multi-site location Affreightment | | 0,25 |
| Multi-site location with both Storage and Transshipment of feed and Road transport of feed. | ≤ 5 products 6-15 products >15 products | 0,25 0,375 0,50 |
| Multi-site location with Storage and Transshipment of feed and/or Road transport of feed and/or Trade in feed. | | 0.50 |
| Multi-site location PO-box | | 0,125 |
| Multi-site location invoicing address | | 0,125 |
| 1 If the multi-site is a tractionair 0,125 day is applicable, up to a maximum of 0,625 day. 2By the number of employees is meant the sum of the number of employees (including part time employees as percentage of FTE) per audited multi-site location. 3 If the multi-site is a PO-Box and/or an invoicing address no additional audit time at the main office is applicable | ||
1.2 Additional requirement
A transport company/tractionair can only be certified under multi-site requirements if the transport company/tractionair carries out all the transport of GMP+ assured feed for the main office exclusively. If this is not the case the transport company/tractionair must be independently certified.
Appendix 4B: Multi-site certification for trade to livestock farms
TS 3.1 Trade to livestock farms
For companies which apply TS 3.1 and which have extra storage locations and/or extra sales points or sales outlets, it is possible to make use of this option of multi-site certification.
Two types are distinguished for Distribution Centre (DC):
- DC acts as the only supplier of the brokers. In this case DC can be seen as a part of the sales points and therefore falls under certification for TS 3.1. Multi-site certification is possible.
- DC is one of the suppliers of the brokers. DC acts much more independently with respect to the brokers (and vice versa) as mentioned under a. In this case DC is seen as an “ordinary” trader and must become certified for the scope trade in feed. Multi-site certification is not possible.
1. General requirements
To be eligible for multi-site certification under TS3.1 Trade to livestock farms the multi-site organization must comply with the following criteria:
- The multi-site organization has a main office from which activities are planned and directed
- The multi-site organization has a network of storage locations and/or sales points
- All storage sites and/or sales points fall under the same quality system which is managed from the main office. This quality system must be based on the GMP+ scope(s)and all the multi-site locations must meet the GMP+ requirements;
- The same methods and procedures are used at all multi-site locations.
- All the multi-site locations are included in the programme of internal audits
- Corrective actions may be imposed from the main office on all storage locations and/or sales points
- The main office must demonstrate that it is able to collect data from every multi-site location, to analyse the data and, where necessary, to make changes with respect to:
- System documents and amendments
- Complaints handling
- Corrective actions
- Planning of internal audits and improvement measures
- If the main office is not the owner of the extra storage locations and/or extra sales points, the main office must have a written agreement with the multi-site locations (storage locations and/or sales points) in which they state:
- to sell GMP+ certified feeds directly to the livestock farmers. Selling to other GMP+ certified companies is not permitted;
- that the purchase of GMP+ certified feeds will only take place via the main office;
- to provide full cooperation to the main office with respect to the activities which are described in all the above points of this option.
This agreement must be signed by all the brokers participating in this Multi-site organization and the signed agreement must be present at the main office and must be available for assessment by the auditor.
In addition, all multi-site locations which have signed an agreement must be known to the Certification Body. The size of the random sample can be determined based on this data.
1.1 Certification
In the event of Multi-site certification for TS3.1 the audit frequency for the extra storage locations or extra sales points (with the exception of the main office) may be reduced in accordance with the following schedule:
| Initial certification-, recertification- and (un)announced surveillance audit | |||
| Number of multi-site locations /sales points (without main office) | 1 | 2 | > 3 |
| Number of multi-site locations to be audited | 100% / 3 years | 100% / 3 years | 33% / 3 years |
Minimum audit time to be spent per audit in days:
| | Minimum audit times per audit |
| Extra storage location | 0,125 |
| Extra sales point | 0,188 |
As all storage locations and/or sales points must work in accordance with the same methods and procedures and under the same quality system, the assessment of the documentation can remain limited to verification of the presence of up-to-date documentation and the completeness of the documentation with respect to the multi-site location.
Appendix 5: Audits at another location
Definition:
See F0.2 Definition list.
Road transport of feed:
For the scope Road transport of feed the announced surveillance and recertification audit may also take place at another location than the registered offices of the GMP+ Certified Company. If a company is acting as a main office of a multi-site construction, the audit at another location is not allowed.
The following requirements apply:
- The GMP+ Certified Company falls into the category: 1-5 FTE.
- The GMP+ Certified Company does not have its own working area.
- No changes are applicable to the company location and/or activities.
- The GMP+ Certified Company offers at least 1 loading compartment which is used for GMP+ transport (trailer / semi-trailer, etc.) for checking.
- The alternative location is suitable for carrying out audits:
- Checking of loading compartments causes no hazardous situations for those involved or bystanders.
- If there is a collective check (multiple companies are invited for audit at the same time) then the privacy of individual companies must be guaranteed.
Road transport of feed - mandatory sub-scope tractionair:
For the scope Road transport of feed, mandatory sub scope tractionair (multi-site location), the initial certification audit, announced surveillance audit and recertification audit may also take place at another location than the registered offices of the GMP+ Certified Company.
The following requirements apply:
- No changes are applicable to the company location and/or activities.
- The alternative location is suitable for carrying out audits:
- If there is a collective check (multiple companies are invited for audit at the same time) then the privacy of individual companies must be guaranteed.
Invoicing address:
For all scopes where the “invoicing address” is applicable (see F0.3 Scopes for certification), the announced surveillance audit and recertification audit may also take place at another location than the registered offices of the GMP+ Certified Company.
The following requirements apply:
- The alternative location is suitable for carrying out audits.
- No changes are applicable to the company location and/or activities.
PO Box:
For all scopes where the “PO Box” is applicable (see F0.3 Scopes for certification), the initial certification audit, announced surveillance audit and recertification audit may also take place at another location. During the initial certification audit the Certification Body has the responsibility to establish that the PO Box complies with the definition of a PO Box inF0.2 Definition list.
The following requirements apply:
- The alternative location is suitable for carrying out audits.
- No changes are applicable to the company location and/or activities.
Trade in feed:
For the scope “Trade in Feed” is applicable (see F0.3 Scopes for certification) the announced surveillance audit may also take place at another location than the registered offices of the GMP+ Certified Company.
The following requirements apply:
- The alternative location is suitable for carrying out audits.
- No changes are applicable to the company location and/or activities.
Affreightment of transport of feed:
For the scopes Affreightment of Inland waterway transport, short sea shipping, rail transport, road transport and sea transport, the announced surveillance audit and recertification audit may also take place at another location than the registered offices of the GMP+ Certified Company.
The following requirements apply:
- The alternative location is suitable for carrying out audits.
- No changes are applicable to the company location and/or activities.
Appendix 6: Remote audits
GMP + International establishes 2 types of Remote options of performing audits that can be used by certification bodies when fulfilling the following requirements:
If an audit is performed in combination with a FRA scope than the requirements as stated in this Appendix prevail.
| Aspect | Full Remote Audit | Guided Remote Audit | |
| Applicability | During the regular certification cycle | During extraordinary events | During extraordinary events |
| FSA Scopes |
|
|
|
| Type of audit |
|
|
|
| Specific Requirements | Prior to the audit, the Certification Body must perform and document a risk assessment with at least the following risks that have a significant impact on the audit:
When applicable, the experience of the last remote audit. | Same requirements for the risk assessment prior to the audit, as for Full remote audit during regular certification cycle. | Same requirements for the risk assessment prior to the audit, as for Full remote audit during regular certification cycle.
|
| The Certification body must secure the Guide’s impartiality and keep it as documented information. | |||
| Audit Time
| Appendix 2 of the GMP+ CR2.0
70% of the audit time must be spent connected with the auditee using ICT tools. | Appendix 2 of the GMP+ CR2.0
70% of the audit time must be spent connected with the auditee using ICT tools. | Appendix 2 of the GMP+ CR2.0
70% of the audit time must be spent connected with the auditee using ICT tools. |
| Audit team competences | Auditor competencies as per GMP+ CR1.0 | Auditor competencies as per GMP+ CR1.0 | Auditor competencies as per GMP+ CR1.0 |
| It is the responsibility of the Certification Body to determine if the Guide is competent. During the audit, the Guide is under the responsibility of the GMP+ accepted auditor of the Certification Body and must not draw conclusions during the audit | |||
| General Requirements | Follows the remaining requirements for the certification process as for a regular on-site audit | Follows the remaining requirements for the certification process as for a regular on-site audit | Follows the remaining requirements for the certification process as for a regular on-site audit |
| 1 If a company is acting as a main office for a production location(s), the full remote audit is not allowed. 2 If a company is acting as a main office of a multi-site construction, the full remote audit is not allowed. 3 Excluding the scopes which physically handle feed: Production (all scopes), Storage and transshipment of feed, Road Transport of feed, Rail transport of feed. (also not in combination with one of the other service scopes). | |||



