1. Introduction
An essential part of the Feed Safety Management System is documented information. In this GMP+ document, examples of different documents have been gathered. These sample documents may support a company in the implementation and daily operation of the Feed Safety Management System. The information in this document is exclusively intended for illustrative purposes and as a tool. If the company wishes to include additional matters (for instance about feed safety or about any other topic) it is free to change this or to leave out certain elements
It is emphasized that – in the end – every company is responsible for its own correct and complete implementation of the GMP+ Feed Safety Management System, and is required to demonstrate this in the context of the certification. The use of these examples does not mean that the GMP+ requirements are being met. It is and shall remain up to the certification body to assess whether the requirements are being met.
2. Explanatory information
These examples do not cover all situations. Additions to and improvements of this document are always welcome. If a company has additional information regarding the implementation and maintenance of the Feed Safety Management System, it can notify GMP+ International of this. In consultation it shall be determined how this document can be supplemented with examples and explanation.
The sample documents are often made available by the GMP+ Community. In no way can GMP+ International be held liable for the use of the information provided.
3. Sample documents
3.1. Palm oil protocol
GMP+ R 1.0 states that a GMP+ certified company is permitted to purchase products or services if they
- fall under a GMP+ FSA certificate, or
- fall under a certificate which is accepted as equivalent
Some feed materials can also be purchased from a company with a specific certificate or from a non-certified company under specific conditions, the so called gatekeeper conditions. This also applies to palm oil.
In par. 4.3.5 of GMP+ TS 1.2 Purchase additional requirements for the purchase of palm oil of non-certified origin under gatekeeper conditions are provided and explained.
As a tool with par. 4.3.5, this document provides a number of sample documents that need to be present when delivering palm oil base on the gatekeeper requirements relating to palm oil.
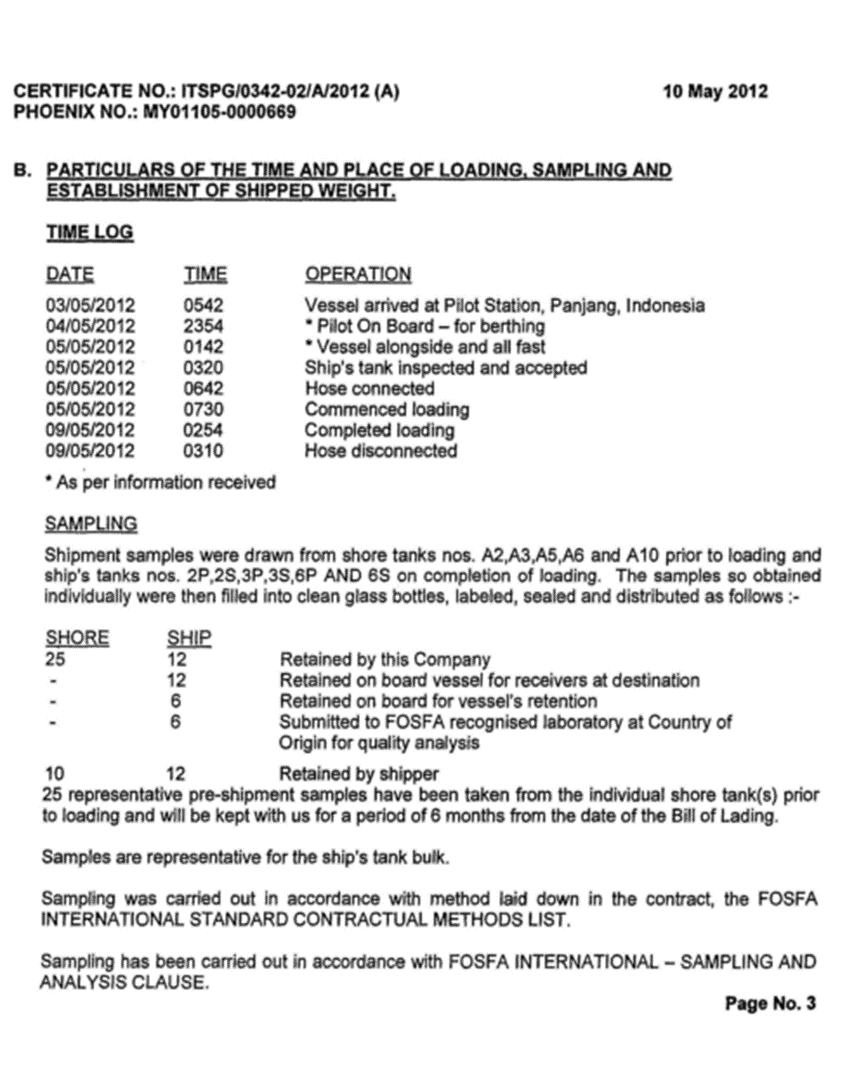
3.1.1. Certificate of Analysis
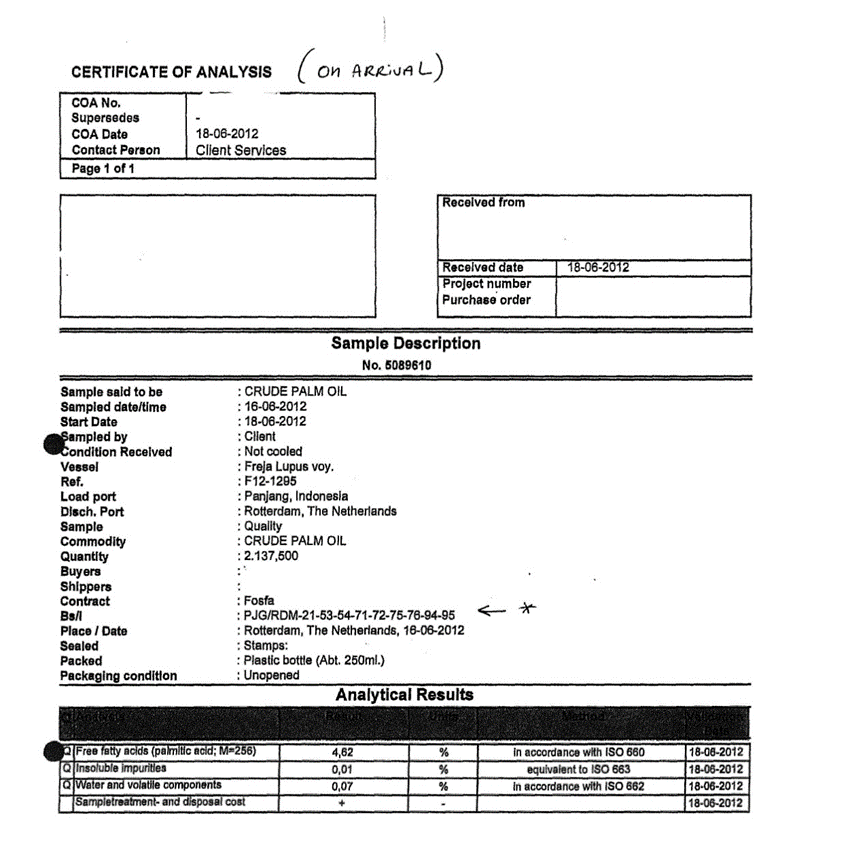
3.1.2. Certificate of of Lading

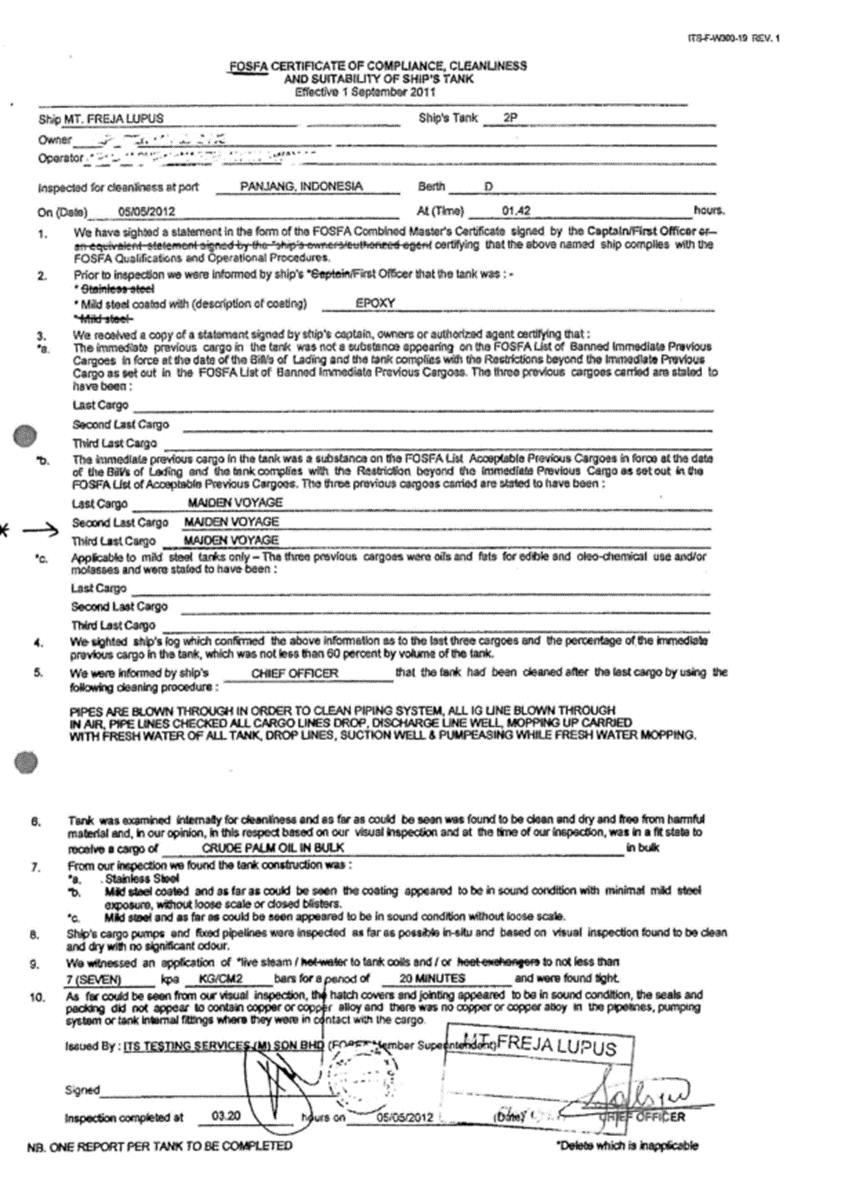
3.1.3. FOSFA, Certificate of Compliance, Cleanliness and suitability of ship’s tank
3.1.4. Sales Contract

3.1.5. Certificate
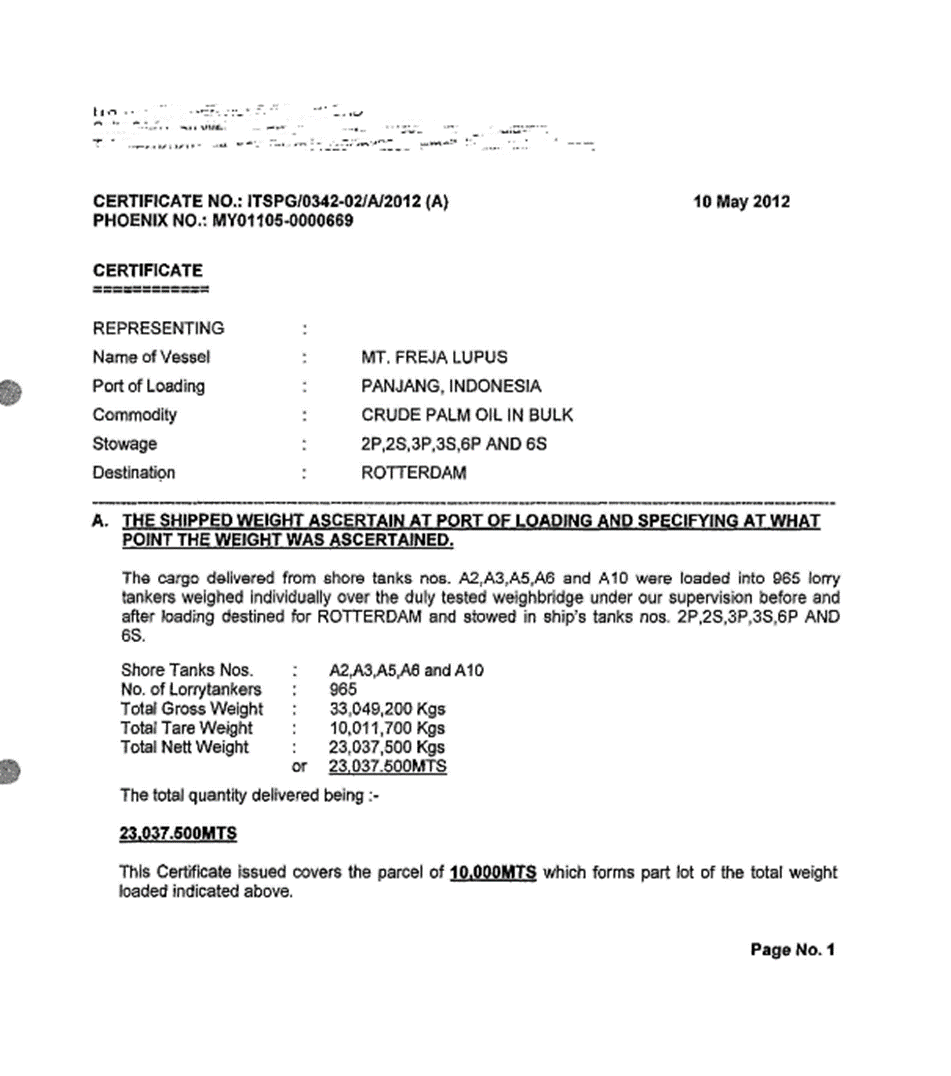
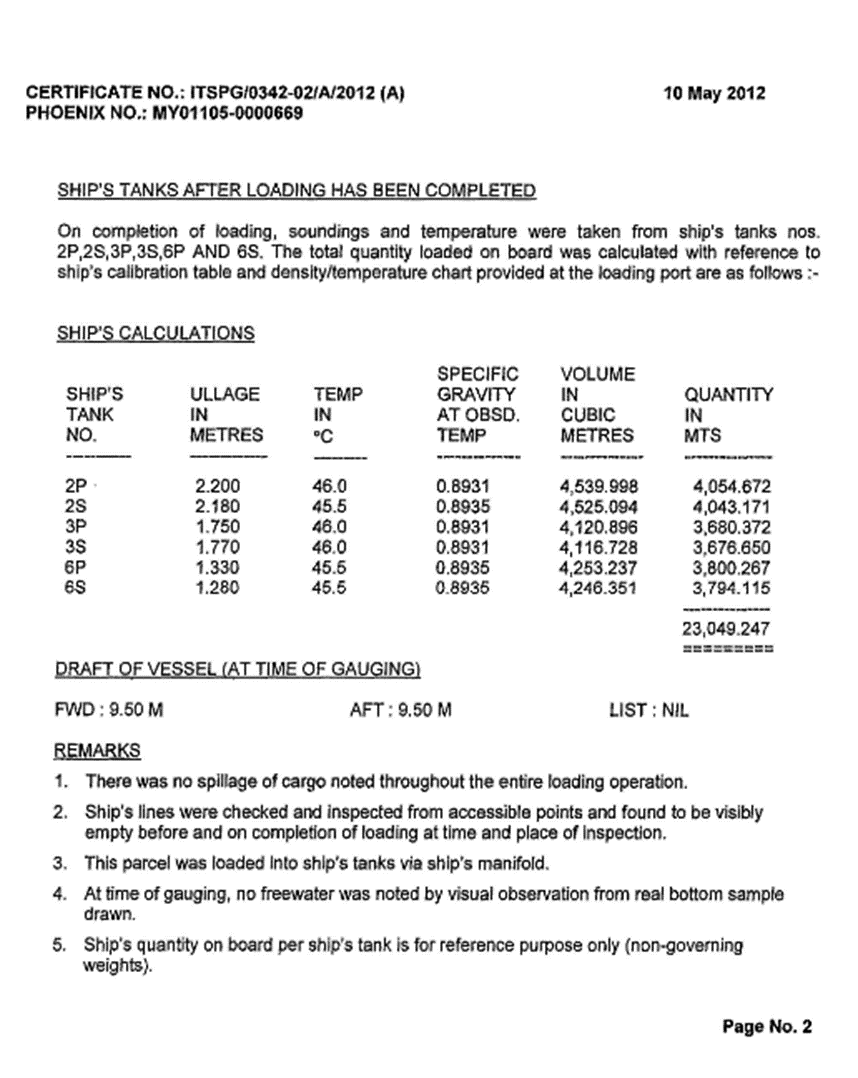




3.2. Load compartments inspections
Load compartment inspections are an important element in the context of assuring safe transport by ships and trains. Below you’ll find several examples of inspection reports.
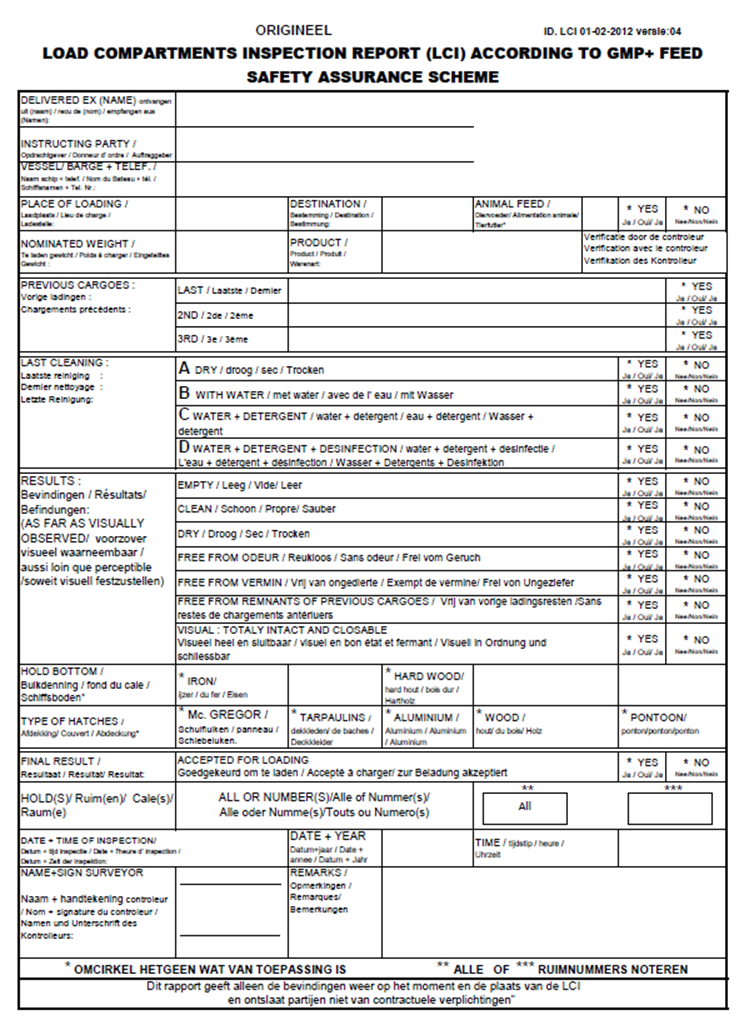

Load Compartments Inspection Report (LCI) according GMP+ Feed Certification scheme.
The following cargo compartments have been inspected in accordance with the GMP+ requirements.

3.3. Purchase of unprocessed agricultural products from grower / grower-collector
GMP+ TS 1.2 Purchase, par. 4.3.1 contains the Gatekeeper protocol for the purchase of unprocessed agricultural products from grower /grower-collector
This concerns the purchase of unprocessed agricultural products for feed and byproducts of the harvest (such as straw and silage). These are purchased from the grower / grower-collector.
In this Gate keeper Protocol is mentioned that the GMP+ certified company must carry out an intensive entry check program, based on its own risk assessment and the quality assurance applied by the grower. Moreover, there must be a quality assurance agreement between the certified company and the grower / grower-collector.
3.3.1. Supplier assessment
An example for documenting a supplier assessment is provided below.
| Supplier assessment – Grower / grower-collector | |
| Version | |
| Grower / grower-collector |
| Contact |
| Address |
| City |
| Phone no./fax |
| E-mail address |
| Assessment date |
Farmyard
* General impression approved / not approved
Storage
* General impression
* Pilotage clean and dry approved / not approved
* Foreign objects approved / not approved
* Pilotage leakage approved / not approved
Silage site
* General impression approved / not approved
* Paved surface approved / not approved
* Cover sheeting undamaged approved / not approved
* Free from visible contamination approved / not approved
Crops
* Weeds visible in crops approved / not approved
* Visible contamination approved / not approved
* Cover sheeting undamaged approved / not approved
* Free from visible contamination approved / not approved
Pest control
* Pest/birds/pets approved / not approved
* Control plan approved / not approved
3.3.2. Quality assurance agreement with grower / grower-collector
The following items should be covered in a quality assurance agreement with grower / grower- collector:
- The control measures which the grower / grower-collector should take.
- All deliveries of unprocessed agricultural products comply with the maximum levels for undesirable substances (e.g. poisonous plants as Colchium automnale or molds) applicable law and regulations for feed and they do not contain any forbidden products, such as: fertilizer, urine, pesticides, animal products, sludge.
- The grower / grower-collector will timely inform the certified company in writing in the event that batches of unprocessed agricultural products fail to meet the information and specifications provided, for instance as a result of a calamity, so that the certified company can take timely action and block batches.
- If any changes occur at the grower / grower-collector causing the above-mentioned to be incorrect, the grower / grower-collector immediately notifies the certified company.
In addition to the above-mentioned items, the certified company and the grower-collector agree at least on the following specific requirements:
- If products are also bought from growers in the area, the grower-collector takes the responsibility to coordinate these requirements with all relevant growers. Demonstrable evidence is provided by the certified company about the education provided to the grower-collector to assure the risks.
- Of all batches, retention samples are taken and retained, that, on request, are available for analysis in case of calamities.
3.4. Complaint form – generic
An important part of the GMP+ Feed Safety Management System is the complaint handling. Complaints may give rise to improve procedures in the system. Below you’ll find an example of a complaint form.
Complaint intended for:
Name:
Address:
City:
Date of complaint:
Date of handling:
| Description of the complaint: |
| Cause of the complaint: |
| Proposed correction: |
| Corrective actions to be taken to prevent repetition: |
| Handling: |
| Response of supplier/buyer: |
3.5. FSDS – Feed Safety Data Sheet
A Feed Safety Data Sheet is intended to provide information in a structured way about the product, the production process and the safety measures used. A model of this is shown below.
Note:
- The model shown is an example. The basic point is that the information should be registered systematically.
- Also other sheets or files may be used, as long as all relevant elements are ad-dressed.
- Possibly not all the information has been provided by the manufacturer in full, certainly not if the feed comes to the end user via a trade channel. In that case each link can add to the information (for example with details of transport, interim storage, etc.).
- This sheet can also be used to report the audit results
| FEED SAFETY SHEET | 0.1. Product | | ||||||||
| 0.2 Status | | |||||||||
| 0.3. Version number | | |||||||||
| 0.4 Version date | | |||||||||
| 1. Responsibility for the feed safety sheet | ||||||||||
| 1.1 | Name of purchasing company (GMP+) | Name | | |||||||
| | Contact | Address: | | |||||||
| Town: | | |||||||||
| Telephone | | |||||||||
| Fax | | |||||||||
| | | |||||||||
| Website | | |||||||||
| 1.2 | Approved by (competent official company) | | ||||||||
| 1.3 | Name of supplying company (non-GMP+ or equivalent) | Name | | |||||||
| | Contact | Address: | | |||||||
| Town: | | |||||||||
| Telephone | | |||||||||
| Fax | | |||||||||
| | | |||||||||
| Website | | |||||||||
| 1.4 | Approved by (competent official company) | | ||||||||
| 2. Identification of the product | ||||||||||
| 2.1. | Product name | | ||||||||
| 2.2. | Trade name | | ||||||||
| 2.3. | Article code of the company | | ||||||||
| 2.4. | Permit number (if applicable, such as product number from TS 1.3 Product list, EU feed material catalogue or EU feed additive register) | | ||||||||
| 2.5. | Product description | | ||||||||
| 2.6. | Origin | | ||||||||
| 2.7. | Supplied by | | ||||||||
| 3. Product description | ||||||||||
| 3.1. | Production process | | ||||||||
| 3.2. | Raw materials and auxiliary substances used (including feed additives and processing aids) | | ||||||||
| 3.3. | Logistical process (transport, (interim) storage, packaging) | | ||||||||
| 3.4. | Storage life | | ||||||||
| 3.5. | Indicative analysis | Parameter | Unit | Average | Min. | Max. | ||||
| | | | | | ||||||
| 4. Standards / Requirements | ||||||||||
| 4.1. | Relevant legislation and other | | ||||||||
| 4.2. | Relevant product standards / | Parameter | Unit | Statutory | Contractual | Internal | ||||
| | | | | | ||||||
| 4.3. | Intended use + reason for destination feed | | ||||||||
| 4.4. | Processing of the product (indicate whether the (former) foodstuff needs further processing or has been processed into feed material) | | ||||||||
| 4.5. | Processing step and instructions for processing | | ||||||
| 4.6. | Storage and retention conditions | | ||||||
| 4.7. | Transport requirements | | ||||||
| 5. Labelling | ||||||||
| | ||||||||
| 6. HACCP | ||||||||
| 6.1. Hazard | 6.2. Risk assessment | 6.3. Control measure | 6.4. Reason | |||||
| Cat. | Likelihood of occurrence | Severity | Risk | |||||
| | | | | | | | ||
| | | | | | | | ||
| | | | | | | | ||
| 7. Monitoring | ||||||||
| 7.1. Para-meter | 7.2. Sampling moment / point | 7.3. Frequency of analysis | ||||||
| | | | ||||||
| | | | ||||||
| | | | ||||||
| 8. Communication in case of non-conformities | ||||||||
| In case the batch does not correspond with the FSDS or the suspicion exist that the health of animals or the food/feed safety is in danger than this must be actively reported to the GMP+ certified company. | ||||||||
| 9. Remarks | ||||||||
| | ||||||||
| 10. Signatures | ||||||||
| ………………………………….. DD / MM / YY GMP+ company (Purchaser) | ………………………………….. DD/ MM / YY Non-GMP+ (or equivalent) certified company (Supplier) | |||||||
| 8. Communication in case of non-conformities | |||||
| In case the batch does not correspond with the FSDS or the suspicion exist that the health of animals or the food/feed safety is in danger than this must be actively reported to the GMP+ certified company. | |||||
| 9. Remarks | |||||
| 10. Signatures | |||||
| ………………………………….. DD / MM / YY GMP+ company (Purchaser) | ………………………………….. DD/ MM / YY Non-GMP+ (or equivalent) certified company (Supplier) | ||||
Explanatory note to the feed safety sheet
| Field | Subject | Explanation |
| 0. | Identification of the feed safety sheet | Field 0 identifies the feed safety sheet. For the purposes of correct identification this field is repeated on each page of the feed safety sheet. |
| 0.1. | Product | Product name |
| 0.2 | Status | The status of the document, for example ‘in concept’, ‘authorized version’, ‘elapsed’. |
| 0.3. | Version number | Version number of the feed safety sheet. |
| 0.4. | Version date | Date on which the version was adopted and put into circulation. |
| 1. | Purchasing and supplying company, | This field identifies the author of the feed safety sheet. This will generally be the producer of the product |
| 1.1 / | Name, address etc. | Identify the organisation which is responsible for the feed safety sheet. |
| 1.3. / | Approved by | Specify the person who authorised the feed safety sheet. |
| 2. | Product identification | Field 2 gives an accurate identification of the product. |
| 2.1. | Product name | Identify the product. Use the designation as prescribed in the legislation. |
| 2.2. | Trade name | State here the usual brand name of the product. |
| 2.3. | Article code | Internal company article number. Specify “n/a” if no use is made of an internal company article number. |
| 2.4. | Permit number | Statutory certification number. State “n/a” if the legislation does not recognise a permit number. |
| 2.5. | Product description | Description of the product, preferably in accordance with the descriptions in the GMP+ Risk assessments. |
| 2.6. | Origin | Describe the origin as accurately as possible. Possibilities are:
|
| 2.7. | Supplied by | If different to 2.6. |
| 3. | Product description | Field 3 describes the characteristics of the product. |
| 3.1. | Production process | Brief but as accurate as possible description of the production process of the product including a flow chart. |
| 3.2. | Used raw materials and auxiliary substances | All the raw materials and auxiliary substances used (including processing aids) |
| 3.3. | Logistical process | Describe the logistical process gone through by the product from the (primary) production up to and including delivery to the end-user. State the method of transport of the product, any (interim) storage and the method of packaging in the various stages in the logistical process. NOTE: the standards and requirements with respect to storage, retention, packaging and transport conditions are described in fields 4.4 and 4.5. |
| 3.4. | Storage life | Indication of the storage life (number of days, weeks, months) of the product (for example, after production). |
| 3.5 | Indicative analysis | This should include a number of relevant characteristics which classify the product. These will generally be non-binding nutritional parameters (such as dry-matter content, raw protein, raw fat, raw cellulose, ash) or the level of active substances (for example in feed additives). |
| 4. | Limits / | Field 4 describes the limits and requirements. |
| 4.1. | Relevant legislation and other requirements. | Summary of the relevant parts of the feed legislation. This may be the applicable European directives and regulations but may also be national legislation and regulations. 'Other requirements' may be specific requirements which apply within the framework of a specific feed safety system in which the customer participates. For example the GMP+ FSA module |
| 4.2. | Relevant feed safety limits / requirements | This relates to the detailed data and not a reference to the legislation or to the GMP+ FSA module. The binding nutritional parameters are included here and also the parameters which are considered to be important in the risk assessment (such as heavy metals in minerals, mycotoxins in grains, PCBs in fats). |
| 4.3. | Intended use | Describe the intended use of the product. For example
|
| 4.4. | Processing instructions | The measures are indicated here which should be taken to be able to use the product correctly and safely. For example:
|
| 4.5. | Storage and retention conditions | Binding requirements for storage and retention. For example:
|
| 4.6. | Transport requirements | Binding requirements for transport. |
| 5. | Labelling | Statement of the way in which the product information is issued. This may be a sample label, a description of the legally-prescribed specifications or an accurate and specific reference to relevant legislation and regulations (a general reference to legislation or regulations is not enough). |
| 6. | HACCP | This field provides a summary of the risk assessment for the product. At least the CCPs (Critical Control Points) are given and also general control measures. |
| 6.1. | Hazard | Precise description of the hazard. |
| 6.2. | Risk Assessment | For the risk assessment one should preferably use the system which is prescribed in the GMP+ FSA module. NOTE: If another system is used then you should indicate this explicitly (in field 8). |
| 6.3. | Control measure | Description of the (specific) control measures which have been established by way of HACCP for the product. |
| 6.4. | Reason | Motivation and argument for the risk assessment, especially with respect to the elements “Likelihood of occurrence” and “severity”. |
| 7. | Monitoring | This field provides a detailed description of the monitoring used in the company (checks, analyses) at the indicated critical points and control measures. |
| 7.1. | Parameter | Describe the characteristic to be examined (for example Aflatoxin B1, Salmonella, Lead, Prussic Acid). |
| 7.2. | Sampling moment / point | Describe the point in the production process where the sample is taken or the inspection takes place (for example free on wagon reception, check before delivery). |
| 7.3. | Frequency of analysis | Describe the frequency at which the monitoring is carried out (for example every batch, 4 times per year, every 10th batch). |
| 8. | Communication in case of non-conformities | |
| 9. | Remarks | |
| 9. | Remarks | Other comments may be placed in this field which are important for this feed safety sheet If a different HACCP system is used than that which is described in the GMP+ FSA module, then this can be described in this field. |
3.6. Appendix to Gatekeeper protocol transport of Hay and Straw
Below you will find an example of an agreement which you can use when applying the gatekeeper protocol Transport, for the transport of hay and straw (GMP+ TS 1.2 Purchase, par. 4.4.1).
| Shipper | |
| Name Shipper | |
| I hereby declare that the loading compartment of this flatbed or curtainsider is free of smell and residue of previous loads. | |
| Date and place | |
| Signature | |
| Transporter | |
| Name transporter | |
| Registration number/ truck- and trailer number | |
| Name driver | |
| I hereby declare that the loading compartment of this flatbed or curtainsider is free of smell and residue of previous loads. | |
| Date and place | |
| Signature | |
| Receiver | |
| Name recipient | |
| I hereby declare that the loading compartment (space) of this flatbed or curtainsider is free of smell and residue of previous loads. | |
| Date and place | |
| Signature | |



