1. Scope of this document
This document contains the acceptation requirements and procedures for Certification Bodies who intend to certify applicant organizations/GMP+ Certified Companies. It also defines the compliance assessment towards Certification Bodies/Critical Locations performed by GMP+ International. In addition the audit times are expressed in days, 1 day equals 8 hours.
2. Normative references
The following documents, in whole or in part, are normatively referenced in this document and are mandatory to comply with. For dated references, only the edition cited applies. For undated references, the latest edition of the referenced document (including all amendments) applies.
- ISO/IEC 17021-1:2015 Conformity assessment – requirements for bodies providing audit and certification of management systems.
- ISO 22003-1:2022(E) Requirements for bodies providing audit and certification of food safety management systems.
- F0.1 Rights and Obligations.
- F0.2 Definition list.
- F0.3 Scopes for certification .
- CR2.0 Assessment and Certification of Feed Safety Assurance scopes.
- CR3.0 Assessment and Certification of Feed Responsibility Assurance scopes .
3. Principles
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 4.1 |
4. Acceptance
4.1. General
A Certification Body that wishes to certify an applicant organization/GMP+ Certified Company according to 1 or more GMP+ scope(s) must demonstrably comply with the requirement laid down in this document in addition to chapter 2. If the Certification Body complies with the requirements GMP+ International will accept the Certification Body in question.
4.2. Acceptation procedure
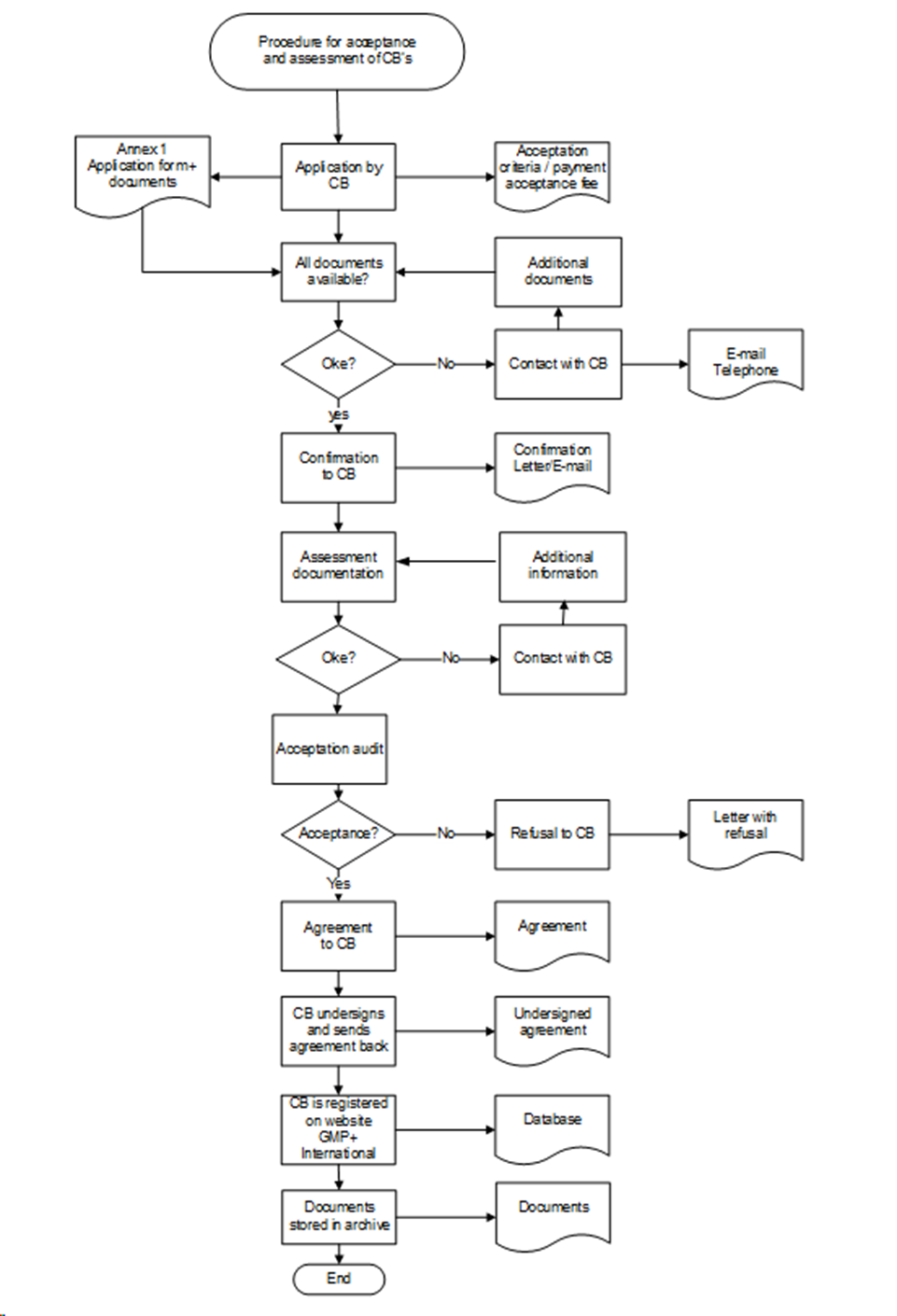
4.2.1. Application
The applicant Certification Body submits an application using the application form published on the website of GMP+ International. The application will be considered when:
- the application form has been filled completely and all the requested documents have been received;
- the application fee for the handling the application has been paid (see CR4.0 Tariffs);
- The applicant Certification Body must have 2 accepted GMP+ auditors/inspectors per scope(s) applied by the Certification Body. The applicant Certification Body must motivate and document its decision accordantly Appendix 2 and must keep all record available for assessment during the acceptation audit.
GMP+ International will confirm this application in writing. If the applicant Certification Body cannot be accepted by GMP+ International within the timeframe of 26 weeks after the first application, GMP+ International will terminate the acceptation procedure. The applicant Certification Body is then not allowed to start a new acceptation procedure within a year after the termination date.
4.2.2. Desk assessment
Desk assessment of the requested documents will take at least 4 and a maximum of 6 weeks. The applicant Certification Body will be informed by GMP+ International about the results. Only after a positive result of the desk assessment an acceptation audit will be performed .
4.2.3. Acceptation audit
An acceptation audit is only possible after a positive result of the desk assessment. The duration of the on-site acceptation audit is at least 1 day.
Audit findings will be addressed in the report to be drawn up and reviewed by GMP+ International. Only when the audit findings are resolved the applicant Certification Body can become accepted.
4.2.4. Applicant Certification Bodies operating with Critical Locations
If, during the application procedure, it is established that the Certification Body operates with Critical Location(s) the following applies:
- Assessment of the Critical Location(s) will be part of the acceptation procedure of the applicant Certification Body.
- For on-site acceptation audit of the Critical Location GMP+ International will charge the applicant Certification Body.
- The acceptance of the applicant Certification Body can only be finalized if the Critical Location(s) complies with the requirements as stated in the GMP+ Feed Certification scheme.
4.2.5. GMP+ Feed Certification scheme License Agreement
If the applicant Certification Body fulfils the requirements of acceptance then:
- GMP+ International will issue a GMP+ Feed Certification scheme License Agreement (Appendix 6 of this document) to the applicant Certification Body to be signed.
- After signing, the Certification Body will send 1 of the original copies back to GMP+ International. The acceptance is complete following receipt of the signed and dated Feed Certification scheme License Agreement.
- GMP+ International will publish the accepted Certification Body and if applicable its Critical Location(s) on the public section of the GMP+ Company Database with a specification for which scopes the acceptance applies (see Appendix 5).
4.3. Acceptation requirements
4.3.1. Accreditation requirements
A Certification Body and its Critical Location(s) must have an accredited food/feed quality management system based on ISO/IEC17021 and ISO22003-1. Except if the Certification Body and its Critical Location(s) certify only the scope of Inland waterway transport and short sea shipping of feed, they must have an accredited management system based on ISO/IEC 17020 and/or operating on an international level in accordance with an approved certification system such as ISO 9001 or equivalent in which Loading Compartment Inspection (LCI) is demonstrated to be part of the certified scope.
4.3.2. Management of impartiality
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 5.2 |
4.3.3. Confidentiality
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 8.4 |
4.3.4. Liability and Financing
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 5.3 |
In addition article 12 of GMP+ Feed Certification scheme License Agreement must apply.
4.3.5. Structural requirements
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 6 |
4.3.6. Resource requirements
4.3.6.1. Competence of personnel
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 7.1 up to and including 7.4 |
| ISO 22003-1:2022(E) | Article 7.1.1 up to and including 7.1.3 |
Additional requirements for the GMP+ coordinator/deputy coordinator, GMP+ auditor, GMP+ Inspector and GMP+ technical reviewer are mentioned in Appendix 1; Certification Bodies must ensure that these requirements are met. The Certification Body must motivate and document its decision according to Appendix 1 and must keep all records available for assessment during the Certification Body audit.
A GMP+ auditor may only conduct GMP+ audits once the GMP+ auditor is accepted for the relevant scope(s) in the GMP+ database.
The GMP+ technical reviewer may only conduct technical review once the GMP+ technical reviewer is accepted for the relevant scope(s) in the GMP+ database.
The Certification Body must appoint 1 person as GMP+ coordinator and can appoint 1 person as GMP+ deputy coordinator for the Certification Body. Application for the acceptance of a GMP+ coordinator and GMP+ deputy coordinator must be submitted to GMP+ International by using the application form as published on the website of GMP+ International.
4.3.6.2. Tasks of GMP+ coordinator/deputy coordinator
The tasks are:
- Contact person to GMP+ International,
- Acceptance of GMP+ auditor, inspector and technical reviewer.
- Issuing audit time reduction.
- Inform GMP+ International of a Critical non-conformity(ies).
- Coordination of examination.
- Ensures that the GMP+ Company Database is op to date (see Appendix 5).
- Internal harmonization, physical harmonization is mandatory with a minimum of once per 2 years. Participation must be documented. Internal harmonization must be demonstrated by means of a presentation/minutes. Harmonization of auditors by the coordinator can be delegated to external parties under the following conditions:
- If the training is outsourced, then the CB coordinator must define the content with the third party to secure sufficient depth.
- The content of the training must be available and verifiable during CB audits.
The Certification Body must establish a procedure in case the GMP+ coordinator/deputy coordinator delegates tasks to an authorized person, excluding tasks 1 up to and including 4.
The position of the GMP+ coordinator/deputy coordinator must be fulfilled by a person connected to the location and the accepted Certification Body.
This means:
- Commitment to the Certification Body and GMP+ Certification Process
- The GMP+ coordinator/deputy coordinator must have a clear and demonstrated commitment to supporting the GMP+ certification process.
- This ensures the GMP+ coordinator/deputy coordinator is actively engaged with the GMP+ certification process and works towards its improvement.
- Knowledge of the Accepted Scopes of the Certification Body
- GMP+ Coordinator/deputy coordinator must have a deep understanding of the GMP+ requirements of the CB’s accepted scope(s) of certification.
- This ensures that the GMP+ coordinator/deputy coordinator can interpret and apply the certification requirements in contexts to maintain consistency and integrity of the certification process and communicates this with the GMP+ auditors and relevant staff members.
- Representative of the Certification Body
- The GMP+ coordinator/deputy coordinator must act as an effective representative of the CB, authorized to ensure compliance with the GMP+ requirements.
- This ensures that the GMP+ coordinator/deputy coordinator must implement corrective and preventive actions ensuring compliance with the GMP+ FC scheme.
- Substantial Influence on Document Management and Internal Processes
- GMP+ Coordinator/deputy coordinator must play an active role and have the authority to review and update the CB’s GMP+ documentation and internal processes and ensures that internal systems for documentation are in place to meet the GMP+ requirements.
- This ensures that the GMP+ documentation is complying with the GMP+ requirements and are fully integrated into the CB’s operational processes.
- Efficient and effective communication
- The GMP+ coordinator/deputy coordinator must be accessible and having a significant presence in GMP+ activities. This includes regular internal meetings, or on-demand consultations to provide clarification on certification matters.
- This ensures efficient and effective communication and secures continuation of the GMP+ certification process.
4.3.7. Information requirements
4.3.7.1. Public information
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 8.1 |
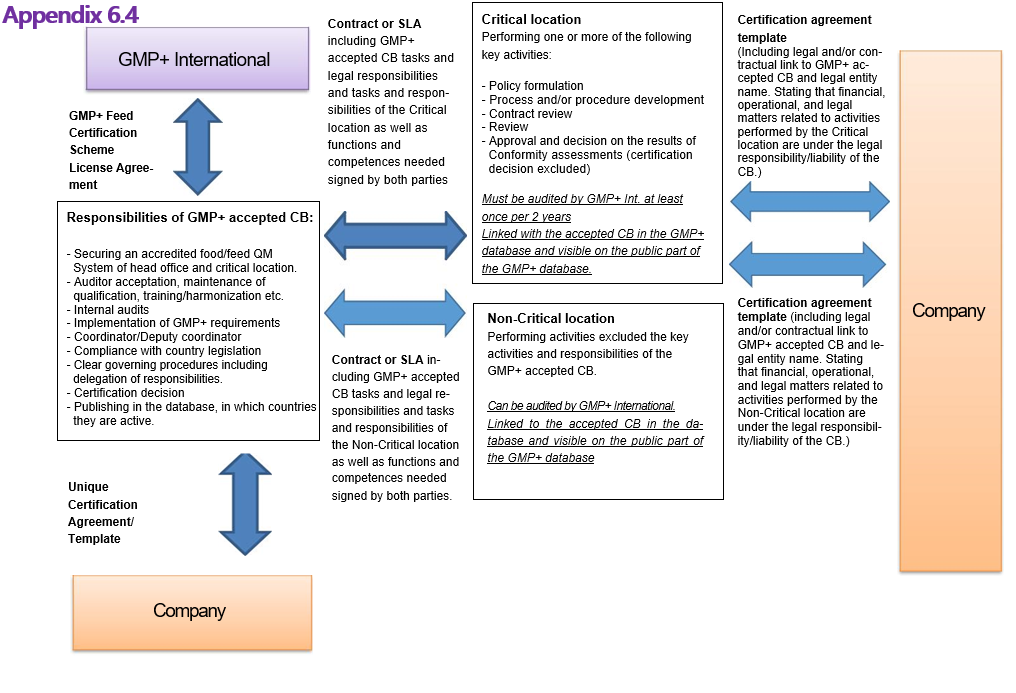
Upon concluding a GMP+ Certification Agreement with an applicant organization , the Certification Body and/or Critical Location must immediately enter the company details as stated in Appendix 5 of this document in the GMP+ Company Database.
In case of a multisite certification or a certification for a tractionair, the main office has to be registered in the GMP+ Company Database and linked to the multi-site location/ traction unit.
After a successful initial audit the scopes of certification must be added.
The Certification Body and/or Critical Location must adapt the GMP+ Database within 2 weeks of any change to the information specified above and in Appendix 5.
If the name, address, and/or registered office/legal entity of the Certification Body or its Critical - and Non Critical Location changes, or in the event of closure, stopping with GMP+ activities the Certification Body is obliged to inform GMP+ International accordingly 3 months in advance.
4.3.7.2. Information exchange between Certification Bodies and its clients.
| Relevant requirements must apply | |
| ISO/IEC 17021-1:2015 | Article 8.5.1 and 8.5.2 |
The Certification Body must retain all relevant certification documentation/information for at least 6 years.
4.3.8. GMP+ International harmonization
GMP+ International organises 2 Feed Safety Assurance (FSA) and 1 Feed Responsibility Assurance (FRA) harmonization meetings per year. Based on the acceptance of the Certification Body, for each meeting the GMP+ coordinator or GMP+ deputy coordinator of the accepted Certification Body must be present. If there are insufficient relevant agenda items for a Certification Body then GMP+ International may decide to issue an individual dispensation from the mandatory attendance.
Each Certification Body is obliged to provide GMP+ International with at least 1 case study for FSA and 1 case study for FRA in a timely manner each year to be discussed during the harmonisation meeting.
4.3.9. Procedures and Documents for GMP+ certification
The Certification Body must have an up to date documented procedure(s) and documents/ templates describing the GMP+ certification process (application through to issuing of the certificate) and handling of EWS notifications. This documented procedure(s) and documents/templates must be part of the quality management system of the Certification Body.
For the control of documented procedure(s) and documents/templates, the Certification Body must address the following, as applicable:
a. distribution, access, retrieval and use;
b. storage and preservation, including preservation of legibility;
c. control of changes (e.g. version control);
d. retention and disposition.
In the event of changes in the certification requirements the Certification Body must implement these at the latest on the implementation date.
5. Compliance assessment
5.1. General
GMP+ International supervises if the Certification Bodies comply with that what is laid down in the relevant CR documents, F documents and the signed license agreement.
The criteria as laid down in this document are used for compliance assessment audits and for determining sanctions.
5.2. Compliance assessment of Certification Bodies, Critical Location(s) and GMP+ auditors/inspectors
The compliance assessment of the Certification Bodies and Critical Location(s) that GMP+ International carries out consists of:
5.2.1. Compliance Desk Assessment
Compliance Desk Assessment is to determine whether the Certification Body and Critical-Non Critical Location(s) comply with the requirements laid down in the GMP+ Feed Certification scheme.
5.2.2. Compliance Audits
The following compliance Audits are applicable:
5.2.2.1. Witness Audits (WA report)
GMP+ International supervises the GMP+ auditors / inspectors by assessing their work method and the way they classify their findings during the execution of their audit. If the GMP+ International auditor observes that a feed safety/feed responsibility risk is not identified during the audit, the GMP+ auditor will be informed by the GMP+ International auditor before the closing meeting in order to confirm whether there is a feed safety/ feed responsibility risk. During a repeat audit/inspection GMP+ International can decide to carry out a witness audit of the auditor/inspector involved.
5.2.2.2. Parallel Audits (PA report)
GMP+ International carries out parallel audits at GMP+ Certified Companies to verify the method by which an audit is planned, executed and reported through the Certification Body. The parallel audit will take place after the audit has been carried out through the Certification Body.
5.2.2.3. CB office Audits (CB report)
GMP+ International will carry out an audit at the Certification Bodies at least once a year to assess implementation of the requirements laid down in the GMP+ Feed Certification scheme. This audit is a full assessment of all requirements. The minimum audit time is 1 day. Reference document for the description of NCR’s is applicable (Appendix 4.2).
5.2.2.4. Critical Location(s) Audit (CL report)
GMP+ International will carry out an audit at the Critical Location(s) at least once every 2 years to assess implementation of the requirements laid down in the GMP+ Feed Certification scheme. The minimum audit time is 1 day.
5.2.2.5. Non-Critical Location(s) Audit (NCL report)
Based on objective evidence from a CB office audit, GMP+ International can carry out a non-critical location audit to assess implementation of the requirements laid down in the GMP+ Feed Certification scheme. The minimum audit time is 1 day.
5.2.3. Analysis
5.2.3.1. Retrospective analysis
Retrospective analysis of the GMP+ Certified Company/GMP+ auditor, which is based on special events and not on a regular basis:
- Retrospective analysis of certification process (RAC report)
It is an analysis of the reports of all Certification Audits and, if available, also of Compliance Audits, conducted at a specific company during the last 36 months.
- Retrospective analysis of a GMP+ Auditor (RAA report)
It is an analysis of the reports of all Certification Audits conducted by a certain GMP+ auditor for a number of reports to be determined by GMP+ International and related to the relevant scope(s).
5.2.3.2. Overall analysis
Overall analysis of the performance of certification (OA report).
It is an annual analysis of performance of a Certification Body during the last 3 calendar years, based on at least:
- Identified nonconformities per GMP+ auditor
- Findings/NCR’s of GMP+ Compliance audits;
- Participation and input in harmonization meetings;
- Exam results of the GMP+ auditors;
- Compliance assessment of the Critical Location(s), if applicable.
The final result of the overall analysis can result in additional compliance assessment for the Certification Body and/or Critical Location(s). The cost for the additional compliance assessment can be charged to the Certification Body. If an action/improvement plan is requested by GMP+ International the Certification Body is obliged to send in such an action/improvement plan within the timeframe requested by GMP+ International.
5.2.4. Examination
Examination of GMP+ auditors, technical reviewers and inspectors is a tool to measure knowledge and application of the knowledge of the GMP+ auditors, GMP+ technical reviewers and GMP+ inspectors in accordance with the requirements of the GMP+ Feed Certification scheme, including the classification of nonconformities.
5.2.5. Report assessment
Based on random samples, GMP+ International will assess GMP+ audit report/checklist on audits carried out through Certification Bodies under the GMP+ Feed Certification scheme. The Certification Body must provide the information immediately on request.
5.3. Identifying and recording findings
Additional to Appendix 4 for the articles 5.2.1, 5.2.2., 5.2.3.1 and 5.2.5 the following applies:
After the compliance assessment has been carried out by GMP+ International the NCR(s) is prepared by the GMP+ International auditor and handed over to the GMP+ coordinator and/or GMP+ deputy coordinator of the Certification Body. GMP+ International is responsible if the observed NCR(s) are justified and well classified. Nonconformities determined during the Critical Location audit will always be issued to the liable Certification Body.
NCR(s) can only be closed if the Certification Body involved conducts a root cause analysis, implements corrective and/or preventive actions and, if applicable, the Certification Body involved must submit objective evidence to GMP+ International.
GMP+ International refers to these actions as Corrective Action Report (hereunder: CAR).
Minor and/or Major nonconformities:
The GMP+ International auditor and GMP+ International (technical) reviewer are responsible for finalizing the compliance report and for assessing the CAR(s) and closing the NCR(s).
Critical nonconformities:
GMP+ International is responsible to make the decision to close and/or upgrade / downgrade the NCR(s) and to make the compliance assessment report final.
5.4. Reporting
For all assessment types as mentioned under Article 5.2 (5.2.4 excluded) a report will be provided to the Certification Body in the English language. In addition the compliance report for all audit types is mentioned in article 5.2. (5.2.4. excluded) can be provided to the Certification Body also in the German or Dutch language.
5.5. Measures and Sanctions
If GMP+ International determines that a Certification Body does not comply with the requirements and obligations of the GMP+ Feed Certification scheme, or the GMP+ Feed Certification scheme License Agreement, it will impose one of the measures or sanctions under a) up to and including e) on the Certification Body. The Certification Body will be informed if necessary by means of an official letter.
- Stating a period of time when the Certification Body/Critical Location must comply with the requirements of the GMP+ Feed Certification scheme. The Certification Body will be asked to provide a corrective action report within a determined timeframe
- Not to renew the GMP+ Feed Certification scheme License Agreement with a Certification Body;
- To suspend the GMP+ Feed Certification scheme License Agreement for a period of maximum 3 months which automatically results that Critical Location and Non-Critical Location are not allowed to conduct any GMP+ activities for the same period;
- To terminate the GMP+ Feed Certification scheme License Agreement possibly after suspension which automatically results in the fact that the Critical Location and Non-Critical Location are not allowed to conduct any GMP+ activities;
- To make the not renewing, suspension and termination as mentioned under b, c, and d publicly known.
During a suspension as mentioned under item c the Certification Body must arrange that all its obligations under the GMP+ Feed Certification scheme are taken over by another GMP+ accepted Certification Body.
As a consequence of not renewing/termination as under item b and d, the concerned Certification Body will be excluded for a period of at least 1 year from participation in the GMP+ Feed Certification scheme. GMP+ International will inform the involved GMP+ Certified Companies.
Appendix 1: Competence of personnel
Appendix 1.1 GMP+ auditor FSA
| Position: GMP+ auditor FSA | ||
| | Applicant auditor | Accepted auditor |
| Education |
| See applicant auditor |
| Knowledge of |
In addition:
| See applicant auditor |
| Audit skills |
| See applicant auditor |
| Audit experience |
and;
|
|
| Working experience |
Exception:
| See applicant auditor |
| Internal harmonization | Each applicant GMP+ auditor must have demonstrably followed an initial training, focussed on the scope(s) in question. |
|
| Examination | Successfully pass the applicable exams. The GMP+ examination regulation and Appendix 2.1 are applicable. | See applicant auditor |
Appendix 1.2 GMP+ auditor FRA
| Position: GMP+ auditor FRA | ||
| | Applicant auditor | Accepted auditor |
| Education |
| See applicant auditor |
| Knowledge of | Knowledge and skill with respect to methods and techniques aimed at the assessment of:
and;
For auditing MI5.1, MI5.2, MI5.3 and MI5.6 the GMP+ auditor must have:
or;
For auditing MI5.4 GMO Controlled the GMP+ auditor must have:
or;
| See applicant auditor |
| Audit skills |
| See applicant auditor |
| Audit experience |
and;
or;
|
|
| Working experience |
| See applicant auditor |
| Internal harmonization | Each applicant GMP+ auditor must have demonstrably followed an initial training, focussed on the scope(s) in question. See “Knowledge of”. |
|
| Examination | Not applicable | Not applicable |
| ¹ Requirements for the trainer: The trainer must have a certificate of the MI5.1, MI5.2, MI5.3, and MI5.6 training provided by GMP+ International or the trainer succeeded for the RTRS endorsed training and must be a GMP+ accepted auditor for one of the applicable FRA scopes. 2 Requirements of the trainer: The trainer must always be in possession of a valid certificate VLOG “Ohne Gentechnik” and must be a GMP+ accepted auditor for MI5.4. | ||
Appendix 1.3 GMP+ technical reviewer FSA/FRA
| Position: GMP+ technical reviewer FSA/FRA | ||
| | Applicant technical reviewer | Accepted technical reviewer |
| Education |
| See applicant technical reviewer |
| Knowledge of |
and
and
In addition FSA:
In addition FRA: Knowledge and skill with respect to methods and techniques aimed at:
and
| See applicant technical reviewer |
| Audit skills | Lead assessor or FSSC Lead assessor training (40 hours minimum, IRCA recognized, or demonstrable equivalent). Not applicable for reviewing TS3.3 Inland waterway transport and short sea shipping of feed | See applicant technical reviewer |
| Review/audit experience |
| Conduct/attend at least 5 audits per scope per year for which the GMP+ technical reviewer has been accepted or assess 5 reports/inspection checklists for the relevant scope. |
| Working experience |
Exception:
| See applicant technical reviewer |
| Internal harmonization | Each applicant GMP+ technical reviewer must have demonstrably followed an initial training, focussed on the scope(s) in question. |
|
| Examination | Successfully pas the applicable exams. The GMP+ examination regulation and Appendix 2.1 are applicable. | See applicant technical reviewer |
Appendix 1.4 GMP+ inspector
| Position: GMP+ Inspector | ||
| | Applicant inspector | Accepted inspector |
| Education | Successfully finished secondary education or at least an equivalent level of experience. | See applicant inspector |
| Knowledge of |
| See applicant inspector |
| Inspection experience | Minimum of 3 on-site inspections as an observer for the relevant scope and/or equivalent scheme as laid down in TS1.2 Purchase. | At least 5 on-site inspections per year
|
| Working experience | Working experience in the feed/ food sector in a relevant position (for example performance of a LCI). | See applicant inspector |
| Internal harmonization | Each applicant inspector must have demonstrably followed an initial training program. | Each inspector must attend at least 8 hours of internal harmonization meeting per calendar year organised by the Certification Body.
|
| Examination | Successfully pas the applicable exams. The GMP+ examination regulation is applicable. | See applicant inspector |
Appendix 1.5 GMP+ coordinator/GMP+ deputy coordinator
| Position: GMP+ coordinator not performing GMP+ audits | ||
| | Applicant GMP+ (deputy) coordinator | Accepted GMP+ (deputy) coordinator |
| Education | Bachelor degree or equivalent level of experience as minimum. | See applicant GMP+ (deputy) coordinator |
| Knowledge of |
and;
and;
and;
| See applicant GMP+ (deputy) coordinator |
| Audit skills | Lead assessor or FSSC Lead assessor training (40 hours minimum, IRCA recognized, or demonstrable equivalent). | See applicant GMP+ (deputy) coordinator |
| Audit experience | 7 GMP+ on-site audits or inspections must be carried out and/or as an observer, equivalent certification schemes as mentioned in TS1.2 Purchaseare applicable. | Per 24 months 7 GMP+ audits/ inspections must be carried out and/or as an observer, equivalent certification schemes as mentioned in TS 1.2 Purchase are applicable. |
| Working experience | Working experience in the feed/ food/responsibility sector in a relevant position (for example quality assurance, production, consultancy on feed safety management systems, laboratory). | See applicant GMP+ (deputy) coordinator |
| Internal harmonization | Each coordinator/deputy coordinator must have demonstrably followed an initial training. | The coordinator/deputy coordinator must attend at least 8 hours of harmonization to a maximum of 24 hours per calendar year depending on the number of scopes where the Certification Body in question is accepted for. This cannot be delegated.
|
| Examination | Not applicable | Not applicable |
Appendix 2: Table of exemptions
In this table the exemptions regarding examination, acceptation and internal harmonization are indicated. Each GMP+ scope listed at the top of table indicates the exemption to the relevant scope in the left column indicated by X. The table does not apply vice versa.
Appendix 2.1 Table of exemptions examination, acceptation and internal harmonization
| | Production of compound feed | Production of premixtures | Production of feed additives | Production of feed materials | Trade in feed | Storage and Trans shipment of feed | Road transport of feed | Affreightment | Road transport of feed & Affreightment | Laboratory testing |
| Production of compound feed1 | | X | | | | | | | | |
| Production of compound feed - petfood Production of compound feed - GMO controlled | X | X | | | | | | | | |
| Production of pre-mixtures1 | X | | | | | | | | | |
| Production of premixtures - GMO controlled | X | X | | | | | | | | |
| Production of feed additives1 | | | | X | | | | | | |
| Production of feed additives - GMO controlled | | | X | X | | | | | | |
| Production of feed materials1 | | | X | | | | | | | |
| Production of feed materials - petfood Production of feed materials - GMO controlled | | | X | X | | | | | | |
| Trade in feed | X | X | X | X | | | | | | |
| Trade in feed - petfood Trade to livestock farms Trade in feed - GMO controlled | X | X | X | X | X | | | | | |
| Storage and Transshipment of feed | X | X | X | X | | | | | | |
| Storage and Transshipment of feed - GMO controlled | X | X | X | X | | X | | | | |
| Road transport of feed - GMO controlled | | | | | | | X | | | |
| Affreightment of short sea shipping Affreightment of inland waterway transport Affreightment of sea transport Affreightment of rail transport | | | | | | | | X | | |
| Affreightment of road transport | | | | | | | X | | | |
| Antibiotic-free production line(s) | X | X | X | X | | | | | | |
| Antibiotic-free production site | X | X | X | X | | | | | | |
| Carbon footprint of feed | X | X | X | X | | | | | | |
| Dioxin-monitoring in feed for laying hens | X | | | | | | | | | |
| QM-Milch | X | X | X | X | X | | | | | |
| RTRS | X | X | X | X | X | | | | | |
| Responsible dairy feed | X | X | X | X | X | | | | | |
| Responsible feed | X | X | X | X | X | | | | | |
| Responsible pig & poultry feed | X | X | X | X | X | | | | | |
| Registered laboratory | | | | | | | | | | X |
| Rail transport of feed | | | | | | | | | X | |
1 Not applicable for examinations
Appendix 2.2 Table of exemptions related to audit frequency
With respect to the retention of acceptance for an auditor/ technical reviewer / inspector insofar the requirement for at least 5 audits per year per scope is concerned, the audits/review/inspection which take place at relevant companies under the accepted certificates as stated in TS1.2 Purchase are applicable in addition the table below may also apply.
| An audit for: | Also applies as an audit for: |
| GMP+ scope:
| GMP+ scope:
|
| GMP+ scope:
| GMP+ scope:
|
| GMP+ scope:
| GMP+ scope:
|
| GMP+ scope:
| GMP+ scope:
|
| GMP+ scope:
| GMP+ scope:
|
| GMP+ scope:
| GMP+ scope:
|
| GMP+ scope:
| GMP+ scope:
|
| GMP+ scope:
| GMP+ scope:
|
| VLOG – ‘Ohne Gentechnik’ Production and Certification Standard: | GMP+ scope:
|
| OQUALIM-STNO Technical Platform “GMO free feed” | GMP+ scope: - Production of compound feed - GMO Controlled - Production of feed materials - GMO Controlled |
| Note: the scopes in the left column of this table apply for the scopes in the right column, but not vice versa. | |
Appendix 3: Procedure for the Acceptance and Assessment of Certification Bodies
Appendix 4: Assessment criteria
Appendix 4.1 Assessment Criteria and Sanctions
Nonconformities are to be classified on the basis of:
- The general assessment criteria as mention in this Appendix
- The sanctions specified must be imposed as a minimum. GMP+ International is allowed to impose stricter sanctions.
CAR(s) needs to be sent to GMP+ Int. at latest 2 weeks before the deadline. GMP+ International is responsible for making the decision to close the nonconformities.
| Classification: Minor nonconformity | |||
| Description | Consequences | Period to close | |
| Certification Bodies
| < 5 nonconformities | Comply with the requirements of acceptance | Within max. 6 months (decided by GMP+ Int.) |
| ≥5 nonconformities | Do not comply with requirements of acceptance | Within 10 weeks | |
| Classification: Major nonconformity | ||
| Description | Consequences | Period to close |
| Certification Bodies
| Do not comply with the requirements for acceptance | within 6 weeks |
| Classification: Critical nonconformity | ||
| Description | Consequences | Period |
| Certification Bodies
| level.1 GMP+ acceptance can be continued only if the NCR is closed | Within 1 week |
| level.2 GMP+ acceptance will be suspended: maximum 3 months | ||
| level.3 GMP+ acceptance will be terminated | ||
| Certification Bodies
| level.1 GMP+ acceptance will be suspended: maximum 3 months | |
| level.2 GMP+ acceptance will be terminated | ||
Appendix 4.2 - Reference document for description of NCR’s for GMP+ accepted Certification Bodies
| Art. Nr. CR documents | Ref. no. | NCR’s CB audits / Compliance Desk Assessment / Report assessment / Retrospective analysis |
| | | GENERAL |
| 1.0 | Non-compliance in assessment of EWS files | |
| CR1.0 art. 1.0 / Appendix 8.1 art.2.9 | 1.1 | Non-compliance in assessment of the contract/SLA with critical/non-critical locations. |
| CR1.0 art. 4.3.9 | 1.2 | Non-compliance regarding internal procedures of the CB. |
| CR1.0 art. 4.3.9 | 1.3 | Documents / QM-system documentation is not up-to-date. |
| CR1.0 art. 4.3.1 | 1.4 | Non-compliance with the accreditation requirement(s). |
| | | CERTIFICATION AGREEMENT |
| CR2.0 art. 5.1.3 / Appendix 2 | 2.0 | No agreement between the CB and GMP+ certified company. |
| CR2.0 art. 5.1.3 / Appendix 2 | 2.1 | The agreement is not secured with the right legal entity. |
| CR2.0 art. 5.1.3 / Appendix 2 | 2.2 | Non-compliance with the minimum obliged audit times. |
| CR2.0 art. 5.1.3 / Appendix 2 | 2.3 | Non-compliance because of re-calculation. |
| CR2.0 art. 5.1.3 / Appendix 2 | 2.4 | The GMP+ requirements as stated in the GMP+ FC scheme are not secured in the agreement. |
| CR2.0 art. 5.1.3 / Appendix 2 | 2.5 | Non-compliance quotation to the companies. |
| CR1.0 art 4.3.2 | 2.6 | Non-compliance impartiality. |
| CR1.0 art. 4.2 / Appendix 8.1 and 8.4 | 2.7 | Non-compliance regarding key activities |
| CR2.0 art. 5.1.3 / Appendix 2 | 2.8 | Non-compliance in the criteria for calculation of the minimum obliged audit times. |
| CR2.0 art.5.1.2 | 2.9 | Non-compliance in application review/pre-transfer review. |
| | | PLANNING, GMP+ COMPANY DATABASE, ROTATION AUDITORS |
| F0.1 art. 4.4 | 3.0 | The GMP+ Company Database is not up to date. |
| CR1.0 art. 4.3.6.1 | 3.1 | Non-compliance auditor acceptation documentation |
| CR2.0 art. 5.1.5.1 | 3.2 | Non-compliance with the rotation requirements |
| CR1.0 art. 4.3.6.1 | 3.3 | Non-compliance with the audit frequency. |
| CR1.0 art. 4.3.6.1 | 3.4 | Non-compliance with the minimum obliged internal harmonization hours. |
| CR2.0 art. 5.2.1 | 3.5 | Non-compliance with audit planning. |
| CR2.0 art. 5.1.3 / Appendix 2 | 3.6 | Non-compliance with audit time reductions. |
| CR1.0 art. 4.3.6.1 | 3.7 | Non-compliance auditor acceptation. |
| CR2.0 art. 5.1.4 and 5.1.6 | 3.8 | Non-compliance audit plan |
| | | CERTIFICATION, REVIEW |
| CR2.0 art. 5.2.4 / Appendix 1 | 4.0 | Incorrect classification of non-conformity. |
| 4.1 | Incorrect certification process. | |
| CR2.0 art. 5.1.2 and 5.2.7 | 4.2 | Non-compliance in the review process. |
| CR2.0 art. 5.2.1 | 4.3 | Non-compliance audit frequency in certification cycle. |
| CR2.0 art. 5.2.8 and 5.2.9.3 | 4.4 | Non-compliance certification decision. |
| | | GMP+ CERTIFICATES/TEMPORARY ACCEPTANCE |
| CR2.0 art. 5.2.9 / CR3.0 art. 4.2.8 | 5.0 | Non-compliance in issued certificates/temporary acceptance. |
| | | ASSESSMENT AND REPORTING |
| CR2.0 art. 5.2.6 / CR3.0 art. 4.2.5 | 6.1 | Non-compliance in reports. |
| CR2.0 art. 5.2.6 / CR3.0 art. 4.2.5 | 6.2 | Non-compliance in handling the report with the GMP+ certified company. |
| | | PARALLEL AND WITNESS AUDITS |
| CR 2.0 art. 5.1, 5.2 and 5.4 / CR 3.0 art. 4.1, 4.2 and 4.4 | 8.1 | Non-compliance in the planning of the audit. |
| CR 2.0 art. 5.2 / CR 3.0 art. 4.2 | 8.2 | Non-compliance of the execution of the audit. |
| CR 2.0 art. 5.2 / CR 3.0 art. 4.2 | 8.3 | Non-compliance of the reporting of the audit. |
Appendix 4.3 - Reference document for the description of findings at GMP+ Certified Companies established during parallel audits.
| Ref. no. | Findings during parallel audits |
| 11.0 | Non-compliance within the context of the GMP+ certified company R1.0; 4.1, 4.2, 4.3, 4.4 |
| 11.1 | Non-compliance within Leadership R1.0; 5.1, 5.2, 5.3 |
| 11.2 | Non-compliance within Planning R1.0; 6.1, 6.2 |
| 11.3 | Non-compliance within Support R1.0; 7.1, 7.2, 7.3, 7.4, 7.5 / TS1.1; 1.0, 2.0 / TS 1.2; 3.0, 4.0 / TS 1.3; 1.0 / TS1.4; 1.0, 2.0 / TS1.8; 1.0, 2.0 |
| 11.4 | Non-compliance within Operation R1.0; 8.1, 8.2, 8.3, 8.4, 8.5, 8.6, 8.7 / TS1.1; 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0 / TS 1.5; 2.0 / TS 1.7; 1.0, 2.0, 3.0, 4.0, 5.0, 6.0 / TS 1.6; 2.0 / TS1.10; 1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 1.10 / TS 1.11; 2.0, 3.0 |
| 11.5 | Non-compliance within Assessment of the FSMS performance R1.0; 9.1, 9.2, 9.3 |
| 11.6 | Non-compliance within Improvement R1.0; 10.1, 10.2, 10.3 |
| 11.7 | Non-compliance within Ordering the transport of feed TS1.9; 2.0 |
| 11.8 | Non-compliance within Affreightment of loading compartments TS1.9; 3.1, 3.2 |
| 11.9 | Non-compliance within Transport of feed TS1.9; 4.1, 4.2, 4.3 |
| 11.10 | Non-compliance within Country Note Dioxin monitoring for poultry feed TS2.1; 2.0, 4.0 |
| 11.11 | Non-compliance within Country Note Antibiotic-free feed TS2.2; 2.0, 4.0 |
| 11.12 | Non-compliance within Country Note QM-Milch TS2.3; 2.0, 4.0, 5.0 |
| 11.13 | Non-compliance within Introduction TS3.1; 1.1, 1.3 |
| 11.14 | Non-compliance within HACCP system requirements TS3.1; 3.1, 3.2, 3.3 |
| 11.15 | Non-compliance within Prerequisites TS3.1; 4.1, 4.2, 4.3, 4.4, 4.5 |
| 11.16 | Non-compliance within Process control TS3.1; 5.1, 5.2, 5.3, 5.4, 5.5 |
| 11.17 | Non-compliance within Introduction TS3.2; 0.1 |
| 11.18 | Non-compliance within Pet food safety management system TS3.2; 1.1, 1.2 |
| 11.19 | Non-compliance within Pre-requisite programmes TS3.2; 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.7, 2.8, 2.9, 2.10, 2.11 |
| 11.20 | Non-compliance within HACCP System TS3.2; 3.1, 3.2, 3.3 |
| 11.21 | Non-compliance within System requirements R5.0; 4.1, 4.2, 4.3, 4.4, 4.5, 4.6 |
| 11.22 | Non-compliance within Supply chain models R5.0; 5.1, 5.2, 5.3, 5.4, 5.5 |
| 11.23 | Non-compliance within Production and Trade of RTRS soy MI5.1; 4.1, 4.2, 4.3, 4.4 |
| 11.24 | Non-compliance within Responsible pig and poultry feed MI5.2; 4.1, 4.2 |
| 11.25 | Non-compliance within Responsible dairy feed MI5.3; 4.1, 4.2 |
| 11.26 | Non-compliance within GMO controlled MI5.4; 4.1, 4.2, 4.3, 4.4, 4.5, 4.6 |
| 11.27 | Non-compliance within Supply chain models MI5.4; 5.1 |
| 11.28 | Non-compliance within Sampling and testing MI5.4; 6.1, 6.2, 6.3 |
| 11.29 | Non-compliance within System requirements MI5.5; 4.0 |
| 11.30 | Non-compliance within Input for the CFP calculations MI5.5; 5.1, 5.2, 5.3, 5.4 |
| 11.31 | Non-compliance within Calculating the CFP of feed MI5.5; 6.1, 6.2, 6.3, 6.4, 6.5 |
| 11.32 | Non-compliance within Additional calculation of emission factors for methane production of feed MI5.5; 7.0 |
| 11.33 | Non-compliance within Compensating the CPF-LUC indicator MI5.5; 8.0 |
| 11.34 | Non-compliance within Informing the customer MI5.5; 9.0 |
| 11.35 | Non-compliance within Production and Trade of responsible feed MI5.6; 4.1, 4.2 |
| 11.36 | Non-compliance within Use of GMP+ FC Logo’s / Trademarks F01; 6.0 |
| 11.37 | Non-compliance within Inland waterway transport and short sea shipping of feed TS3.3; 1a-b, 1.5, 2, 3, 4.2, 5a-b-c, 6, 7, 8, 9a-b-c, 10, 11, 12, 13, 14, 15, 16, Annex 1 |
| 11.38 | Non-compliance within Laboratory testing TS4.1; 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0, 12.0, 13.0, 14.0 |
| 11.39 | Non-compliance within Registered laboratories TS4.2; 2.0, 3.0, 4.0, 5.0 |
Appendix 5: Responsibility for processing data into the GMP+ database and/or entitlement to publish
| Responsible for: | Article | GMP+ Int. | CB/Critical Location |
| Publication of CB/Critical Location | 4.2.5 | X | - |
| Publication of a suspended CB/Critical Location | 5.5 | X | - |
| Publication of the termination of the GMP+ Feed Certification scheme License Agreement | 5.5 | X | - |
| Publication that GMP+ International will not renew the GMP+ Feed Certification scheme License Agreement | 5.5 | X | - |
| Publication that another CB takes over the obligations when the original CB is suspended | 5.5 | X | - |
| Inform involved GMP+ Certified Companies when the acceptance of a CB is withdrawn/not renewed. | 5.5 | X | - |
| Publication of a suspended Company | 5.3 (CR2.0) | X | - |
| Publication of the withdrawal of a GMP+ Certificate of a Company due to noncompliance | 5.3 (CR2.0) | X | - |
| Responsible for: | Article | GMP+ Int. | CB/Critical Location |
| Processing data of GMP+ Certified Company (visiting address) to be published on the public part of the GMP+ portal | |||
| Official name of GMP+ Certified Company | 4.3.7.1 | - | X |
| Street | 4.3.7.1 | - | X |
| Number | 4.3.7.1 | - | X |
| Zip code | 4.3.7.1 | - | X |
| City | 4.3.7.1 | - | X |
| Country | 4.3.7.1 | - | X |
| Business legal registration number / number Chamber of Commerce | 4.3.7.1 | - | X |
| Phone number | 4.3.7.1 | - | X |
| E-mail address | 4.3.7.1 | - | X |
| Website | 4.3.7.1 | - | X |
| Ship name | 4.3.7.1 | - | X |
| EU number | 4.3.7.1 | - | X |
| Processing data of GMP+ Certified Company (postal address) to be published on the public part of the GMP+ portal | |||
| PO Box number | 4.3.7.1 | - | X |
| Zip code | 4.3.7.1 | - | X |
| City | 4.3.7.1 | - | X |
| Country | 4.3.7.1 | - | X |
| Certification data of GMP+ Certified Company to be partly published on the public part of the GMP+ portal | |||
| Scope(s) | | - | X |
| Certified since | | - | X |
| Start date of certificate | | - | X |
| End date | | - | X |
| Date suspension (if applicable) | | - | X |
| Date withdrawal (if applicable) | | - | X |
| Reason of suspension (if applicable) | | - | X |
| Date suspension lifted | | - | X |
| Reason of withdrawal (if applicable) | | - | X |
| Certification status | | - | X |
| Audit time reduction | | - | X |
| Responsible for: | Article | GMP+ Int. | CB/Critical Location |
| Certification data of GMP+ Certified Company to be partly published on the public part of the GMP+ portal | |||
| Linking/Unlinking of a multisite location/traction unit to head office/principal | | - | - |
| Contact person at GMP+ Certified Company | | - | X |
| Emergency telephone number (24/7 number) | | - | X |
Appendix 6: GMP+ Feed Certification scheme License Agreement
This Appendix contains the model of the license agreement which will be used by GMP+ International to define the individual GMP+ Feed Certification scheme License Agreement for each Certification Body as mentioned in article 3 of F0.1 Rights and Obligations.
The goal of this document is to provide legal framework for all parties involved in the GMP+ Feed Certification scheme, visualized in Appendix 8.4 of this document. Enabling transparency for all parties involved the following main objectives were determined:
- establish a contractual link, starting from GMP+ International up to and including to the GMP+ certified company;
- Compliance assessment toward Certification Bodies can be carried out only by GMP+ International auditors
To establish this, the criteria laid down in this document are based as much as possible on international standards and including the GMP+ requirements.
Appendix 6.1: Model agreement
The following text must be used for the GMP+ Feed Certification scheme License agreement (hereafter License Agreement) between GMP+ International and an accepted Certification Body.
Beginning (model) agreement:
_________________________________________________________________________
The undersigned:
- The Dutch law limited liability company GMP+ International BV, with its registered office at the Braillelaan 9 in (2289 CL) Rijswijk (The Netherlands), registered at the Trade Register of the Dutch Chamber of Commerce under number 27364542,
(hereinafter: “GMP+ International”),
and
- [Name of the certification body], with its registered office at the [address, including country], registered at the [official name of local trade register where the entity is registered] under number [ ],
(hereinafter: “Certification Body”),
(hereinafter collectively referred to as “the Parties”)
Whereas:
- GMP+ International is the holder of rights to the GMP+ Feed Certification scheme, an international certification scheme covering the whole feed chain, consisting of the GMP+ Feed Safety Assurance Module for the assurance of feed safety and the GMP+ Feed Responsibility Assurance Module for the assurance of feed responsibility.
- The GMP+ Feed Safety Assurance Module integrates a variety of feed safety requirements into one module, such as requirements for the feed safety management system, HACCP, product standards, traceability, monitoring, prerequisites programs, chain approach and the Early Warning System. The GMP+ Feed Responsibility Assurance Module incorporates requirements for production, trade, storage & transshipment, and transport of feed products with respect for humans, animals and the environment;
- GMP+ International holds rights to the Licensed IP, (definitions are described in Article 1 below);
- The certification of the GMP+ Feed Certification scheme is not performed by GMP+ International but by licensed Certification Bodies. Companies wishing to obtain GMP+ Feed Certification scheme certification directly approach such a licensed certification body;
- The Certification Body is involved in the certification and is interested in obtaining a License Agreement to perform certification according GMP+ Feed Certification scheme and using the Trademarks, Logos and Documentation;
- GMP+ International is interested in granting the Certification Body a License Agreement, with the aim to allow the Certification Body to certify companies complying with the scope(s) of the GMP+ Feed Certification scheme.
Now it is agreed between the Parties as follows:
- Definitions
For the purpose of this Agreement, the definitions in the GMP+ Feed Certification scheme are applicable. See F0.2 Definition list, the Feed Safety Assurance module and the Feed Responsibility Assurance module.
In addition or notwithstanding, the following terms and definitions shall have the meaning within the framework of this Agreement as set forth below:
1.1 Appendix(es): the Appendixes attached to this agreement which form an integral part of this agreement and have been separately initialed by the Parties and in which the agreements between the Parties have been detailed.
1.2 Basic Payment: an annual payment, consisting of two components: a) a basic tariff, and use of database web application and API, and b) a variable payment depending on the number of scopes and kind of activities of the Certification Body, and if applicable, its Critical Location and the GMP+ Certified Companies certified by the Certification Body.
1.3 Critical location: a location of Certification Body conducting one or more key activities (for definition key activities see Chapter 3 of F0.2 Definition list).
1.4 Documentation: any documentation provided to the Certification Body by GMP+ International during the term of the License Agreement, including but not limited to the documents of the GMP+ Feed Certification scheme.
1.5 GMP+ Certified Company Emergency Telephone Number: a telephone number of the GMP+ certified company which can be reached 24/7 and 365 days of the year in case of emergencies.
1.6 GMP+ CompanyDatabase: a publicly accessible database administered by GMP+ International and actualized by GMP+ International, Certification Bodies and/or Critical Location containing details of the Certification Bodies, Critical Locations and GMP+ Certified Companies.(See Appendix 5 of this document)
1.7 Licensed IP: Trademarks, Logos and the Documentation.
1.8 Logos: any logo of GMP+ International that is protected or not by a trademark in the countries of activity of the users. See chapter 5 of F0.1 Rights and Obligations.
1.9 Measure(s): has the meaning as defined in article 5.5 of this document.
1.10 Non-Critical location; a location of a Certification Body conducting no key-activities.
1.11 Sanction(s): has the meaning defined in Article 5.5 of this document.
1.12 Suspension: the Certification Body is temporarily suspended for a maximum period of 3 months, if GMP+ International rules that the Certification Body’s is in breach of this License Agreement and therefore denied the rights arising from this License Agreement. This suspension automatically results that Critical Location and Non-Critical Location not being allowed to conduct any GMP+ activities for the same period. All remaining requirements and obligations are stated in Article 5.5 of this document.
1.13 Termination: To terminate the License Agreement under the conditions as set out in GMP+ Feed Certification scheme.
1.14 Trademarks: the trademarks licensed to GMP+ International, listed in Appendix 6.2 of this document.
1.15 Website: GMP+ International’s website www.gmpplus.org.
- The GMP+ Feed Certification scheme
2.1 Upon signing of this License Agreement, the Certification Body guarantees that it implements and complies with all applicable requirements in the GMP+ Feed Certification scheme. Parties agree that the most recent version of the GMP+ Feed Certification scheme is an integral part of this License Agreement.
2.2 The most recent version of the GMP+ Feed Certification scheme is publicly accessible at the Website www.gmpplus.org of GMP+ International. Upon request of the Certification Body, GMP+ International shall promptly provide the Certification Body with a free copy of the most recent version of the GMP+ Feed Certification scheme, electronically or otherwise. By signing this License Agreement, the Certification Body expressly agrees to the above ways to take note of the GMP+ Feed Certification scheme and declares that prior to signing this License Agreement it has read and understood these documents.
2.3 GMP+ International may at any time amend the GMP+ Feed Certification scheme. GMP+ International shall promptly, electronically or otherwise, notify the Certification Body of amendments to the GMP+ Feed Certification scheme. The certification body must comply with the amendments of the requirements within a period, as mentioned in the S9.1 List of changes GMP+ FC scheme 2020, unless GMP+ International determines a shorter period.
2.4 Upon signing of this License Agreement the Certification Body and its Critical Location(s) must have the required accreditation see article 4.3.1 of this document.
2.5 The Certification Body must provide full cooperation to GMP+ International in the accurate implementation of the GMP+ Feed Certification scheme.
2.6 GMP+ International is allowed to conduct Compliance Assessments at the premises of the Certification Body and its, Critical-, Non-Critical Location(s) as well as at the GMP+ Certified Companies. The Certification Body and its Critical-, Non-Critical Location(s) must lend its full cooperation to such Compliance Assessments. See article 5 of this document.
2.7 GMP+ International shall, as far as reasonably possible, enable the Certification Body to give advice with respect to proposed changes to the GMP+ Feed Certification scheme via its public consultation procedure and the GMP+ Subcommittee Certification & Compliance.
2.8 The Certification Body has the right to nominate candidates to represent all Certification Bodies for membership of the GMP+ Subcommittee Certification & Compliance.
2.9 The Certification Body can only delegate key activities to Critical Location(s) and non-key activities to Non-Critical location(s) by means of a Contract or a Service Level Agreement (SLA).
2.10 The Certification Body shall keep proper records of Contracts and/or SLA established between the Critical/Non-Critical location(s), and shall have these records readily available for assessment by GMP+ International during Compliance Assessment.
2.11 The Certification Body must inform GMP+ International immediately in case a Critical/Non-Critical location is in breach of the Contract and/or SLA.
- Grant of license
3.1 Subject to the terms and conditions of the License Agreement, GMP+ International grants and the Certification Body accepts, a non-exclusive license to certify companies complying with the scope(s) of the GMP+ Feed Certification scheme.
3.2 Subject to the terms of the License Agreement, GMP+ International allows the Certification Body to use the GMP+ Logo/Trademarks as further set out in F0.1 Rights and Obligations. The right to use the GMP+ Logo/Trademarks can exclusively be granted by GMP+ International. The right to use the GMP+ Logo/Trademark can be withdrawn if the Certification Body does not comply with the requirements as set out in the GMP+ Feed Certification scheme and fails to remedy the same within the determined timeframe.
3.3 The Documentation shall not be published nor modified in any way by the Certification Body. The Certification Body has the right to reproduce the Documentation for its own use or, subject to the conditions of the License Agreement, to make it available to the GMP+ Certified Companies.
3.4 The Certification Body has the duty to immediately report to GMP+ International any infringement of the Licensed IP which comes to the notice of the Certification Body.
3.5 GMP+ International shall always have the right to sue in respect of infringement of the Licensed IP without the Certification Body, at its own expense and under its sole liability, and to earn exclusively the results of the proceedings.
3.6 The Certification Body must perform and document its internal audits (at the Critical location) to be conducted every 12 months.
3.7 The Certification Body is responsible to comply with the applicable country legislation were the Certification Body is located.
3.8 The Certification Body is responsible for:
- the certification decision;
- auditor acceptation, maintenance of qualification, training etc.;
- clear governing procedure including delegation of responsibilities;
- have a GMP+ coordinator/ deputy connected to the location and the Certification Body.
- Certification and auditing of Companies
4.1 The Certification Body shall conclude a unique Certification Agreement and/or Certification agreement template with a Company before conducting an Initial (Certification) Audit. During the validity of a GMP+ certificate, the Certification Body must conduct audits at the GMP+ Certified Company in accordance with the GMP+ Feed Certification scheme.
4.2 After the decision of the Certification Body, the Certification Body/Critical location shall have the right to issue Certificates to Companies for the scopes specified in Appendix 6.3.
4.3 The Critical/Non-Critical locations may offer GMP+ International’s activities to the local market only on behalf of the Certification Body which must always mention the name and logo of the Certification Body.
4.3.1 In the standardized certification agreement signed on behalf of the Certification Body in the form of a template approved by the Certification Body, between the Critical/Non-Critical location and the Company a legal or contractual link to the Certification Body and legal entity name must be included, stating the financial-, operational- and legal matter related to activities performed by the Critical/Non-Critical location are under the liability of the Certification Body.
4.3.2 The reports issued to the GMP+ Certified Companies shall contain the name and address of the GMP+ International accepted Certification Body without the logo of the Critical and/or Non-Critical location. However the report may make reference to the contact details of the Critical and/or Non-Critical location issuing the report in question.
4.3.3 The certificate issued to the GMP+ Certified Company shall contain the name and address of the Certification Body without the logo of the Critical Location. However the certificate may make reference to the contact details of the Critical location issuing the certificate in question. The certificate issued shall not create any confusion as to the Certification body.
4.4 The Certification Body, Critical/Non-Critical Location is obliged to keep proper records of unique- and/or standardized Certification Agreement in the form of a template approved by the Certification Body, and results and reports of the Audits at GMP+ Certified Companies and the certificate, and is obliged to have these records readily available in the global quality management system for Compliance assessment by GMP+ International. In case GMP+ International wants to receive (copies of) records, the Certification Body, Critical/Non-Critical Location is obliged to make the requested information available to GMP+ International accordingly.
4.5 The Certification Body must inform GMP+ International immediately in case a GMP+ Certified Company is in breach of the Certification Agreement with respect to conditions and obligations arising from the GMP+ Feed Certification scheme.
4.6 GMP+ International has the right, at any time, to conduct a Compliance Audit of the GMP+ Certified Company or to participate as witness during an Audit. The cost of these audits is at the expense of GMP+ International.
- Confidentiality
5.1 The Certification Body must not disclose to third parties any Documentation, or use it for any purpose other than as described herein, unless GMP+ International agrees otherwise prior to disclosure in writing.
5.2 Non-disclosure obligations arising from Article 5.1 shall not apply to Documentation the contents of which have become generally known or easily accessible or which have been lawfully revealed by a third party. In case to comply with law and/or legal regulation and/or by orders of a court, governmental agency but always with prior notice to GMP+ International.
5.3 The Certification Body must procure that all of its employees and Critical/Non-Critical location and their employees, if any, adhere to the obligations arising out of Article 5.1.
5.4 With exception of the cases of authorization mentioned in the GMP+ Feed Certification scheme, GMP+ International shall not disclose to third parties any information of the Certification Body and will not use it for any purpose other than as described herein, unless the Certification Body agrees otherwise prior to disclosure in writing.
- Fees
6.1 Every year, the Certification Body must pay to GMP+ International the Basic payment. The amounts hereof are specified in the CR4.0 Tariffs of the GMP+ Feed Certification scheme. The amounts specified therein are agreed net. If VAT is applicable, this shall be borne by the Certification Body. Any local and/or other taxes, governmental fees or dues, if applicable, shall also be borne by the Certification Body.
Every year, the Critical Location must pay to GMP+ International a fixed payment as establish in article 2.2 of the CR4.0 Tariffs.
6.2 The Basic Payment is determined by GMP+ International. GMP+ International reserves the right to unilaterally adjust the amounts in the CR4.0 Tariffs of the GMP+ Feed Certification scheme.
6.3 The Certification Body/Critical location must keep the GMP+ Company Database up to date as mentioned in Appendix 5 of this document in order to enable GMP+ International to extract the necessary information required to calculate the Basic payment.
6.4 In addition to the Basic Payment, the Certification Body hereby agrees to pay GMP+ International a fee for the examination by GMP+ International of its auditors. The amounts hereof are specified in the CR4.0 Tariffs of the GMP+ Feed Certification scheme.
The amounts specified therein are agreed net. If VAT is applicable, this shall be borne by the Certification Body. Any local and/or other taxes, governmental fees or dues, if applicable, shall also be borne by the Certification Body.
- GMP+ Company Database
7.1 The Certification Body must comply with the (applicable) requirements and obligations as stated in Article 4.3.7.1. of this document which is an integral part of this agreement.
- Default
8.1 In the event the Certification Body, Critical/Non-Critical location is not or not fully performing one or more of the obligations arising from this Agreement, including but not limited to obligations arising from the GMP+ Feed Certification scheme measures and sanctions as stated in Article 5.5 of this document, which is an integral part of this agreement,will be imposed.
- Conditions for the GMP+ accepted Certification Body operating with Critical and Non-Critical Location(s).
9.1 The Certification Body and its Critical and Non-Critical location must operate under the same management and the same digital global quality management system accessible for all locations.
9.2 The Certification Body shall have the means to substantially influence and control the activities of the locations. The Certification Body shall be able to demonstrate that such influence and control is in place and properly working.
9.3 The Certification Body maintains the final responsibility for the GMP+ International activities performed by the Critical, Non-Critical location.
9.4 Where the Critical location(s) carry out key activities then the GMP+ International accepted Certification Body shall in its contract and/or SLA clearly identify the address of these locations.
9.5 The use of Critical and/or Non-Critical locations is only allowed for locations within the same organization and where the Certification Body maintains the legal responsibility for the activities performed and certificates/reports issued by the Critical and/or Non-Critical locations.
The legal responsibility must be demonstrated on the basis of contract/SLA or equivalent legal relationships between the Certification Body and the Critical and/or Non-Critical locations and internal regulations in the organization that further specify these relationships in terms of management and legal responsibilities.
9.6 Using Critical and/or Non-Critical locations is possible for all types of locations such as subsidiaries, branches, agencies, offices, etc. regardless of their legal personality, as long as they carry out clearly defined and relevant activities within the scope(s) of the GMP+ Feed Certification scheme.
9.7 Holding the final responsibility as mentioned in article 9.4 for activities performed by the Critical and/or Non-Critical location, implies that the Certification body takes the operational, financial and legal responsibility/liability for activities performed by these locations, and this operational, financial and legal responsibility/liability must be stated in the GMP+ certification agreement with its customers.
- Duration and termination
10.1 This Agreement will enter into force on the date of signature by the Parties and will remain in force until 15 July 20XX.
10.2 GMP+ International is entitled to terminate this Agreement with immediate effect by written notice to the Certification Body if:
- the Certification Body does not comply with the binding instructions issued by GMP+ International as stated in Article 5.5 of this document.
- the Certification Body has no accredited food/feed quality management system.
- the Certification Body does not or not fully perform one or more of the essential of its obligations arising from the GMP+ Feed Certification scheme.
10.3 Either Party may terminate or not to renew this Agreement with immediate effect by written notice to the other Party if:
- either Party commits any breach of any of the provisions of this Agreement and, in the case of a breach capable of remedy, fails to remedy the same within a determined timeframe after receipt of an official letter giving full particulars of the breach and require corrective actions;
- an encumbrance takes possession or a receiver is appointed over any of the property or assets of that other Party or is declared bankrupt;
- that other Party makes any voluntary arrangement with its creditors or becomes subject to an administration order;
- that other Party goes into liquidation;
- anything which, under the law of any jurisdiction, is analogous to any of the acts or events specified in clauses 11.3 a)-d) of this Agreement; or
- that other Party ceases, or threatens to cease, to carry on business.
10.4 In the event that a Certification Body terminates or not to renew the License Agreement they are obliged to inform all parties concerned three months in advance to enable all GMP+ Certified Companies to transfer to another Certification Body.
- Liability
11.1 The Certification Body shall reimburse GMP+ International for the principal amount of a claim for compensation or damages by a GMP+ Certified Company and/or a Company directed at GMP+ International insofar as GMP+ International’s liability towards the GMP+ Certified Company and/or the Company is related to the performance of the Certification Agreement by the Certification Body and subsequently its Critical/Non-Critical location and on the condition that such liability has been established by a final court judgment or final arbitral award.
11.2 The indemnity as set out in Article 11.1 does not apply if:
- A claim directed at GMP+ International is based on acts of GMP+ International itself (including but not limited to use of the binding instruction, a violation by GMP+ International of the GMP+ scheme or external communication by GMP+ International)
- Or the claim is based on such facts or circumstances as the Certification Body and subsequently its Critical/Non-Critical location did not know or could not have been expected to know and taken into account at the time of the performance of the Certification Agreement.
11.3 The indemnity as set out in Article 11.1 applies nonetheless if an act of GMP+ International as set out in Article 11.2 is due to GMP+ International having based its conduct on incorrect information provided by the Certification Body and/or Critical/Non-Critical location (and the Certification Body and/or Critical/Non-Critical location knew or have known that it was incorrect).
11.4 In case of a claim within the scope of this Article 11, GMP+ International shall forthwith fully inform the Certification Body and not enter into an amicable settlement with claimant without prior written consent of the Certification Body, on penalty of forfeiture of the rights under this Article 11.
11.5 The Certification Body shall at all times be fully liable towards GMP+ International for all acts and omissions by its Critical/Non-Critical location.
11.6 The liability of parties towards each other in connection with performance of this Agreement and this Article 11 is at all times limited to € 250,000 per claim with a maximum of € 1,000,000 per calendar year.
- Miscellaneous
12.1 This Agreement constitutes the complete and full agreement between the Parties.
12.2 Any invalidity of individual provisions of this Agreement shall not affect the validity of the remaining provisions of this Agreement. The remaining provisions of this Agreement shall remain in full force and effect and enforceable to the fullest extent permitted by law. Any provisions found to be invalid or unenforceable shall be substituted by such other provisions coming, in a legally permissible way, as close as possible to the economic meaning and intention of such invalid provision.
12.3 The Certification Body is not allowed to assign this Agreement in whole or in part or any benefit or interest therein.
- Applicable law and disputes
13.1 This Agreement shall be governed by and construed in accordance with the laws of The Netherlands.
13.2 All disputes arising in connection with the Agreement, or further contracts resulting therefrom, shall be heard by the District Court of Rotterdam, having exclusive jurisdiction.
Drawn up and signed in duplicate,
| GMP+ International BV | [Name Certification Body] |
| Managing Director | [Name of legal representative] (Title of legal representative) |
| …………………………… (Signature) | …………………………… (Signature) |
| Place: Rijswijk | Place: ....................................... |
| Date: ……………………… | Date: ……………………… |
Appendix 6.2: Trademarks and Logo’s
Trademarks and applicable logo(s) will be added in individual Agreement(s)
The trademarks have been protected globally. For instance, GMP+ International is the owner of the following registrations:
GMP+ FSA word mark: Benelux, EU and international registrations
GMP+ FSA logo: Benelux, EU, UK and international registrations
GMP+ FRA logo: EU and UK registrations
A complete overview of the list will be shared upon request.
Appendix 6.3: Scopes covered by the GMP+ Feed Certification scheme (License) Agreement
This document is part of the GMP+ Feed Certification scheme License Agreement which has been entered into force <date><month><year> for the period until <date><month><year> between GMP+ International and
| Name of the Certification Body | : | |
| Address | : | |
| Location | : | |
The GMP+ Feed Certification License Agreement will relate to the following scopes of the GMP+ Feed Certification scheme with effect from the date specified below:
| GMP+ scopes | Accepted / Not accepted |
| Production of compound feed | |
| Production of compound feed | |
| Production of compound feed – petfood | |
| Production of premixtures | |
| Production of premixtures | |
| Production of feed additives | |
| Production of feed additives | |
| Production of feed materials | |
| Production of feed materials | |
| Production of feed materials – petfood | |
| Trade | |
| Trade in feed | |
| Trade in feed – petfood | |
| Trade to livestock farms | |
| Storage and Transshipment | |
| Storage and Transshipment of feed | |
| Transport | |
| Road transport of feed | |
| Rail transport of feed | |
| Inland waterway transport and short sea shipping of feed | |
| Affreightment | |
| Affreightment of short sea shipping | |
| Affreightment of inland waterway transport | |
| Affreightment of sea transport | |
| Affreightment of rail transport | |
| Affreightment of road transport | |
| Laboratories | |
| Laboratory testing | |
| Registered laboratory | |
| Additional scopes | |
| Antibiotics-free production site | |
| Dioxin-monitoring in feed for laying hens | |
| QM-Milch | |
| FRA scopes | |
| RTRS | |
| Responsible dairy feed | |
| Responsible pig & poultry feed | |
| Production of compound feed – GMO controlled | |
| Production of premixtures – GMO controlled | |
| Production of feed additives – GMO controlled | |
| Production of feed materials – GMO controlled | |
| Trade in feed – GMO controlled | |
| Storage and Transshipment of feed – GMO controlled | |
| Road transport of feed – GMO controlled | |
| Carbon footprint of feed | |
| Responsible feed | |
Valid until: <date><month><year>
GMP+ International B.V.
Managing Director
[Name Certification Body]
…………………………….
[Name of legal representative]
Managing Director
……………………………
(Signature)
………………………………...
Date:………………………
Appendix 6.4: Contractual links