1. General monitoring requirements
The requirements listed in this document are in addition to those set out in the R1.0 Feed Safety Management Requirements document.
1.1. Monitoring plan
Information (like EWS, RASFF or other signals about possible hazards) that might influence the established monitoring plan must be assessed. If necessary, the monitoring plan must be adapted immediately.
The GMP+ certified company may make use of representative monitoring results from other companies (for example: suppliers). This particularly applies to monitoring results for undesirable substances where the level theoretically speaking no longer changes, such as heavy metals, pesticides, dioxin.
Note: ‘representative’ does not necessarily mean: ‘from the delivered batch’.
If there is any doubt, uncertainty or unclearness about the representativeness of the monitoring results from other companies, the certified company must verify on the representativeness.
The GMP+ certified company must determine if specific monitoring requirements described in this document are applicable and must therefore be implemented in the monitoring plan of the certified company.
In case of overlap between different monitoring requirements in this document or other GMP+ FSA documents, the GMP+ certified company must apply the strictest monitoring requirements.
Helpful tip
Special attention should be given to the representativeness of the
- monitoring results received from suppliers (for example: qualifications of the laboratory; used method; detection limit);
- sampling and;
- samples (for example: correct method; do they really represent the feed).
1.2. Monitoring frequency
The monitoring frequency must give assurance that all identified hazards and determined risks remain under control.
The GMP+ certified company must follow the minimum monitoring frequencies as established in the monitoring protocols in this document. If the hazard analysis shows that additional monitoring is needed, the GMP+ certified company must increase the monitoring frequency in accordance with the result of the hazard analysis.
Helpful tip
Remember that, the monitoring frequency (on a motivated determined period. See §1.1)of feed materials can be calculated using the following formula:

| Variable | Explanation | ||||||||||||||||||||||
| Frequency | The number of samples to be monitored (on a motivated determined period) | ||||||||||||||||||||||
| Volume | Volume in tons of feed materials per year. In principle, the number of samples to be monitored is based on the quantity of feed material which is produced, traded, processed or stored. As the quantity of feed material increases, the number of samples per ton will decrease. Kilograms must be assumed for some feed materials for which, on a motivated determined period, only a small quantity is produced, traded or processed. | ||||||||||||||||||||||
| Likelihood of occurrence | The default value for likelihood of occurrence is 1. The GMP+ certified company may raise or lower this value if reasons are given. The following considerations may apply to this:
It is up to the certified company to decide that the likelihood of occurrence value can be lowered. The certified company must select a likelihood of occurrence value which is below one on the basis of (historical) monitoring results. The following must be kept in mind:
For some undesirable substances the monitoring results for an area can be considered to be representative while, for other undesirable substances, only monitoring results for the same production location is representative.
| ||||||||||||||||||||||
| Severity | This factor expresses the degree of harmfulness of an undesirable substance. For the value for severity use can be made of information of the Feed Support Products (FSP): Severity is great factor 5 Severity is moderate factor 3 Severity is small factor 1 This leads to the following factors:
| ||||||||||||||||||||||
Note:
| |||||||||||||||||||||||
1.3. Sampling
Unless the monitoring protocols in this document state otherwise, the GMP+ certified company must take samples in accordance with the requirements as laid down in document TS1.6 Sampling.
1.4. Analysing
1.4.1. Analysis method
The analysis must be carried out by a laboratory approved for this under the GMP+ FSA module. See TS1.2 Purchase.
1.4.2. Sharing analysis results
The results of the analyses that are carried out within the framework of applying the monitoring protocols in this document must be uploaded in the GMP+ Monitoring database. The analysis results must be uploaded within a month from the date of issuance of the Certificate of analysis and shared anonymously with the GMP+ Community in the GMP+ Monitoring database.
Helpful tip
Be aware to enter the correct value (in mg/kg). A laboratory might report the results in ppb’s. Be sure to check on this. If so, please divide this result by 1000 before entering in the database. Example: 3 ppb = 0.003 mg/kg.
Helpful tip
The GMP+ certified company who makes use of analysis results from other companies (for example suppliers) should not enter the results into the GMP+ Monitoring database.
1.4.3. Collective monitoring plan
It is possible for GMP+ certified companies to carry out their monitoring plan together (in a collective monitoring plan). The following requirements must be applied with respect to this option:
- The collective monitoring plan must comply with the GMP+ requirements.
- The scope of the monitoring plan must be established (‘which feed is included’) and which companies are participating.
- The collective monitoring plan must be representative for the feed which the manufacturers produce, trade, treat and / or process. Its representativeness must be motivated.
- All the participating companies must obtain all the relevant sampling and monitoring results.
2. Monitoring protocol of Aflatoxin B1 in maize and by-products of maize
2.1. Scope
This protocol gives specific requirements for sampling and analysing Aflatoxin B1 in:
- maize, processed or unprocessed and
- by-products of maize,
which will be delivered in the GMP+ chain.
2.2. Application
This protocol applies to the GMP+ certified company which trades or processes the products referred to in § 2.1.
The GMP+ certified company may make use of analysis results of the supplier, provided that these results represent the delivered batch.
2.3. Requirements per classification of countries of cultivation
The GMP+ certified company must comply with the requirements associated with the risk classification of the countries of cultivation of the maize. To consult the risk classification of countries of cultivation see Classification of countries of cultivation.
The country of cultivation of the maize must always be known in order to apply the protocol correctly.
In case the country of cultivation is not known, the GMP+ certified company must apply the requirements of the risk classification High.
For maize from Low-risk countries, the following requirements apply:
- Every GMP+ certified company is responsible for monitoring the aflatoxin level according to its own monitoring plan, in accordance with the HACCP principles as laid down in the GMP+ standard.
- Sampling in accordance with the requirements as laid down in document TS1.6 Sampling.
- All links in the chain, including the end user, must be periodically informed (on request) about the analysis results.
For maize from Medium and High risk countries, the requirements laid down in the following table apply.
| | High | Medium | ||
| Means of delivery | All means of transport or storage location excl. direct delivery by trucks | Direct delivery by trucks* | All means of transport or storage location excl. direct delivery by trucks | Direct delivery by trucks* |
| Responsible for application of protocol | First GMP+ certified company in the supply chain | | GMP+ certified company that receives the trucks. | |
| A representative sample must be taken from each batch for analysis. | |||
| Batch size |
|
|
|
|
| Sample taker | Independent supervisory organization:
| In accordance with the requirements as described in document TS1.6 Sampling . | Independent supervisory organization:
| In accordance with the requirements as described in document TS1.6 Sampling |
| Sample method |
In case of direct ship-to-ship transshipment: see below (Notes) | According to general GMP+ FSA requirements TS1.6 Sampling | ||
| Place/moment of sampling |
| |||
| | |||
| Frequency of analysis | Each final sample. | |||
| Sample preparation | The GMP+ certified company responsible for application of this protocol must arrange with the laboratory to prepare the sample in accordance with the following conditions:
The remains of the final sample must be retained for re-analysis. | |||
| Validity of the certificate of analysis | The certificate of analysis must demonstrate that the sampling was conducted no more than 3 months before the date of delivery. In case of stored batches and re-analysis after 3 months, the highest measured Aflatoxin B1 value (from all sampling moments) should be taken as the value since it is not obvious that Aflatoxin B1 content could decrease over time. All analysis results applicable to the batch (including the expired results) must accompany the batch. | |||
| | |||
| Information to the client and end user** | Certificate of analysis / analysis report There must be a clear link between the delivered batch and the certificate of analysis / analytical report The country of cultivation | |||
| Positive release | The end user must be informed about the results of the analysis before unloading unless seller and customer agree otherwise. In any case results must be available before using as or processing in feed. | |||
| | | For maize from medium risk countries and only in case of direct delivery by trucks: This maize may be processed in all feed except dairy feed. The results do not necessarily have to be available before processing, but can be received later. Note: This requirement may be different for other accepted schemes. | ||
| | |||
| Direct ship-to-ship transshipment | In case of direct ship-to-ship transshipment (from a seagoing vessel, coaster, inland waterway vessel to an inland waterway vessel) the method described in “GAFTA sampling rules No. 124” is permitted under the following conditions:
| |||
| Specific addition for (food producers of) maize by-products | In principle, the produced maize by-product must be sampled and tested. However, as an alternative the incoming maize may be tested. The food producer must establish a monitoring protocol for the incoming maize provided that
The following information must be available and shared on request with the customer:
This information must demonstrate that the maize by-product does not exceed the limits laid down in TS1.5 Specific feed safety limits. Note: The food producer may participate in other schemes with mutual recognition with different requirements about monitoring and sharing of information. If so, it is recommended that contractual arragements are made about the provision of information. | |||
| * Direct delivery by trucks: this refers to previously untested maize that is delivered by truck, for example from growers or non-certified collectors ** End user: the end user is the GMP+ FSA certified company that delivers (compound) feed to the farmer (= the final link in the GMP+ chain) | ||||
3. Monitoring protocol of Salmonella in feed
3.1. General requirements
3.1.1. Scope
This protocol contains minimum monitoring requirements for Salmonella in feed.
Excluded from this scope are feed products in which Salmonella cannot survive due to the intrinsic properties of the feed products: the pH value, temperature and/or low water activity (Aw-value). The exclusion must be based on a documented validation.
Helpful tip
More information about Salmonella and the conditions under which Salmonella cannot survive can be found in the GMP+ factsheet Salmonella. This factsheet is found on the GMP+ International Portal.
3.1.2. Application
This protocol applies to the GMP+ certified company that:
- produces feed, or
- outsources the production of feed to another company. See TS1.2 Purchase for the requirements for purchasing production or processing on a contract basis.
If responsibilities with regard to the application of this monitoring protocol are transferred to another company, this must be kept as documented information.
3.2. Monitoring frequency
The GMP+ certified company must ensure that the feed does not exceed the Salmonella limits, as laid down in TS1.5 Specific feed safety limits.
The ongoing effectiveness of the control measures must be monitored in accordance with the below-mentioned minimum monitoring frequencies. Monitoring is done by analysing representative samples taken of the end-products as close as possible to the end of the production line.
Helpful tip
Please remind that when having positive Salmonella results you may decide to increase the monitoring frequency.
3.2.1. Compound feed for poultry
The GMP+ certified company that produces compound feed for poultry must sample and analyse the compound feed according to the monitoring frequency as specified in the following table:
| Type of compound feed per target animal | Minimum number of samples to be analysed | Minimum number of samples when a validated control measure is applied* |
| Breeding animals kept as grandparents or great-grandparents | 1 per 48 tons | 1 per 144 tons |
| Chickens or turkeys reared for breeding animals other than grandparents and great-grandparents | 1 per 120 tons | 1 per 360 tons |
| Chickens or turkeys kept for breeding purposes | 1 per 240 tons | 1 per 720 tons |
| Broilers, laying hens and animals reared for laying hens | 1 per 480 tons | 1 per 1440 tons |
| Meat turkeys | 1 per 720 tons | 1 per 2160 tons |
| *The validation must be kept as documented information. | ||
Helpful tip
Be aware that the reduction of the monitoring frequency may not be possible due to national feed related legislation.
Helpful tip
A validated control measure is a control measure that has proven to be effective in controlling Salmonella in feed. Heat treatment and acidification are well known and often-used control measures.
3.2.2. Compound feed (except feed for poultry)
The GMP+ certified company that produces other compound feed than those intended for poultry must sample and analyse the compound feed at least once per 10,000 tons.
3.2.3. Feed materials
The GMP+ certified company that produces feed materials must sample and analyse each feed material according to the monitoring frequency as specified in the following table:
| Annual production of feed material | Minimum number of samples to be analysed | Minimum number of samples when a validated control measure is applied* |
| Less than or equal to 50,000 tons | 8 per year | 2 per year |
| More than 50,000 tons | 20 per year | 5 per year |
| *The validation must be kept as documented information. | ||
Helpful tip
See helpful tips 1 and 2 in § 3.2.1
3.2.4. Feed additives and premixtures
The GMP+ certified company that produces feed additives or premixtures must sample and analyse the feed additives and premixtures based on HACCP.
3.3. Analysing
Salmonella-positive results must be serological classified. See R1.0 Feed Safety Management Systems Requirements for the requirements on how to handle non-conform products.
4. Monitoring protocol of animal protein
4.1. General requirements
4.1.1. Scope
This protocol contains minimum monitoring requirements for tissue proteins from mammals in compound feed, including wet mixes, for ruminants.
4.1.2. Application
This protocol applies to the GMP+ certified company that produces compound feed, including wet mixes, for ruminants.
4.2. Monitoring frequency
Monitoring is done by analysing the samples taken of end-products on the presence of tissue proteins from mammals in accordance with the below-mentioned minimum frequency:
| Production in tons per year | Minimum number of samples to be analysed |
| Less than 10,000 | 1 per quarter |
| Between 10,000 and 40,000 | 2 per quarter |
| More than 40,000 | 3 per quarter |
5. Monitoring protocol of Oils and Fats as regards dioxin and dioxin like PCB’s
5.1. General requirements
5.1.1. Scope
This protocol provides specific requirements for monitoring the levels of dioxin and dioxin-like PCB’s in oil and fat products, which
- originate from the processing of oil seed, oil refining, animal fat processing and/or fat blending, and;
- are used in feed, and;
- are produced, traded, stored, transported or used by GMP+ certified companies.
5.1.2. Application
This monitoring applies to GMP+ certified companies that produce or trade the products mentioned under § 5.1.1..
GMP+ certified companies are exempt from monitoring if they dispose of an analysis result, covering the purchased batch (unique reference of the batch must be included in the analysis report).
5.2. Monitoring frequency
It is important to highlight that the monitoring frequencies, as is specified in the following tables, are not meant to substitute the individual feed business operator’s HACCP system, and do not exempt a feed business operator from applying the HACCP principles, which includes the establishing of an adequate monitoring plan. This monitoring plan must, at least, include the minimum monitoring frequency laid down in the following tables as follows:
| Class | 1 | 2 | 3 | 4 |
| Product | Not allowed for feed. Included in the tables for reason of transparency and completeness See also TS1.4 Forbidden Products and Fuels | Product for use in feed | Product for use in feed | Product for use in feed |
| Monitoring frequency | Not applicable. | 100% monitoring with a Positive Release. One analysis per batch (max.1000 tons | One representative analysis per 2000 tons or 5000 tons (with a minimum of one representative analysis per year) | Based on the company’s internal hazard analysis |
| Rationale | Products are forbidden for feed | The presence of dioxins and dioxin-like PCB’s is possible | The presence of dioxins and dioxin-like PCB’s is unlikely | The presence of dioxins and dioxin-like PCB’s is highly unlikely |
The labeling of feed materials that fall under this monitoring must – where possible – use the names listed in Regulation (EU) no. 68/2013 (European Catalogue of feed materials).
Such a name ensures that the product is identified with certainty and to determine the monitoring (class 1, 2, 3 or 4) to which this feed material has been subjected with maximum precision.
In case the name used is not included in Regulation (EU) no. 68/2013, only monitoring conform product class 1 (forbidden products) or product class 2 can be applied.
Class 3 or class 4 monitoring can only be applied for products of which the name is included in the European Catalogue of feed materials and for which a product class 3 or 4 has been identified in the tables above.
Helpful tip
See Appendix 1 for a list of relevant products with names, description and EU catalogue numbers
The monitoring must be carried out in accordance with the class as specified in the table below:
| How to read | |
| EU Food | A producer that is registered (according to art. 6 of Reg. (EC) No. 852/2004) as an EU food operator. |
| Other | A producer not registered (according to art. 6 of Reg. (EC) No. 852/2004) as an EU food operator. |
| Table 1: Products of vegetable origin | EU food | Other |
| See TS1.4 Forbidden products and fuels for oil/fat products not allowed in feed | 1 | 1 |
| Fatty acids distillates (13.6.5) | 2 | 2 |
| Deodistillates, treated | 2 | 2 |
| Acid oils from chem. refining (13.6.1) | 4 | 2 |
| Fatty acids esterified with glycerol (13.6.2) | 4 | See Appendix 1 |
| Mono, di and tri glycerides of fatty acids (13.6.3/13.6.9) | ||
| Salts of fatty acids (13.6.4) | ||
| Crude fatty acids from splitting - excluding fermentation of organic matter, and enzymatic interesterification of oil (13.6.6) | ||
| Pure distilled fatty acids from splitting - excluding fermentation of organic matter, and enzymatic interesterification of oil (13.6.7) | ||
| Sucrose esters of fatty acids (13.6.10) | ||
| Sucroglycerides of fatty acids (13.6.11) | ||
| Glycerin (13.8.1/13.8.2), Lecithin (2.21.1) and Gums | 4 | 4 |
| Used filter aids/use bleaching earth | ||
| Soap stocks (13.6.8) | ||
| Vegetable oil/fat, crude and refined except for crude coconut oil (2.20.1) | ||
| Crude maize germ oil (1.2.13) | ||
| Crude coconut oil if supplied as feed material (2.20.1) | 2 | 2 |
| Oils/fats recovered from food business operators (2.20.2) | 2 | 2 |
| Other oil/fats products derived from a biodiesel production process out of a non-refined feedstock | 2 | 2 |
| Imported tocopherols extracted from vegetable oil and tocopheryl acetate made thereof | 2 | 2 |
| Table 2: Products of animal origin | |
| See TS1.4 Forbidden products and fuels for oil/fat products not allowed in feed | 1 |
| Animal fat from land animals | |
| Animal fat processors, edible fats and oils, (Regulation (EC) 853/2004) (9.2.1) | 3 |
| Cat. 3 operators, fats and oils, (Regulation (EC) 1069/2009) (9.2.1) | 3 |
| Acid oils (13.6.1) & soap stocks | 3 |
| Deodistillates, treated | 2 |
| Fatty acid distillates (13.6.5) | 2 |
| Fat from gelatin production | 2 |
| Product from fish oil processing | |
| Crude fish oil (10.4.6) | 2 |
| Fish oil, produced from fisheries with no monitoring history, from unspecified origin or from the Baltic Sea (10.4.6) | 2 |
| Fish oil, from fish by-products from non-EU approved establishments manufacturing fish for human consumption (10.4.6) | 2 |
| Fish oil, produced from blue whiting or menhaden (10.4.6) | 2 |
| Products derived from fish oil which is neither refined nor listed in this table (including fish oil refinery by-products ) | 2 |
| Soap stocks (13.6.8) and acid oils (13.6.1) from fish oil | 2 |
| Refined fish oil (and all other fish oils not specified above) (10.4.6) | 3 |
| Table 3: Products from fat blending | |
| See TS1.4 Forbidden products and fuels for oil/fat products not allowed in feed | 1 |
| Incoming products | See tables 1 and 2 |
| or | |
| Outgoing blends of fats/oils | 2 |
Note: Instead of monitoring incoming batches according to these classifications, a fat blender may choose to monitor 100% of outgoing batches (= class 2). This choice needs to be declared to the auditor. EU-located feed business need also to declare the choice to the competent authority.
5.3. Positive release
To comply with the Positive Release requirements, GMP+ certified companies (producers and, if appropriate, traders, see § 5.1.2) within the supply chain, may use several systems. In this section, a number of systems, are explained. These systems are allowed to be used by GMP+ certified companies, active within the supply chain. However, if the competent authority, or a customer, has additional requirements, these must also be satisfied.
The analysis results of dioxins and dioxin-like PCBs must be available, before any use in feed materials such as compound feed and premixtures.
Note: with ‘shipped’ is meant that the product is transported from the producer’s facility to (for example) a storage tank, located at the customer’s facility. The producer, still owns the product and is therefore responsible for the product. With ‘delivered’ is meant that the product is not only transported to the customer, but also the ownership of the product is transferred to the customer.
| No. | Option | Remarks |
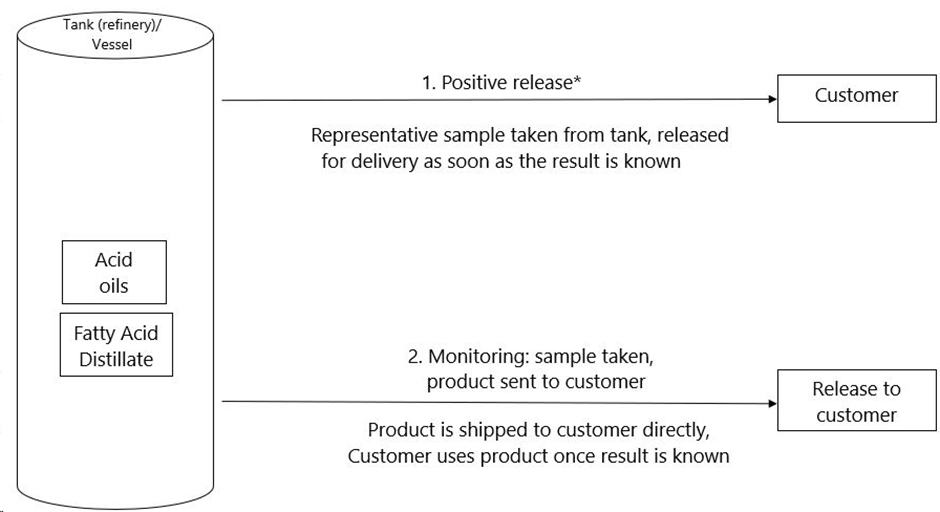
| 1 | The producer, takes a representative sample of the product located at his storage tank, he then sends the sample to a laboratory for the analysis of dioxin and dioxin-like PCBs. The product is shipped, and delivered to the customer, once the test results are known, and are within the specifications. | For more details as regards sampling and analysis, see § 1.3 and §1.4. Customer must be informed of the results, through means of an Analytical Report. |
| 2 | The producer takes a representative sample of the product, located at his storage tank, he then sends the sample to a laboratory for an analysis as regards dioxin and dioxin-like PCBs. Meanwhile, the product is shipped to the customer. The actual delivery of the product (transfer of ownership) will take place once the dioxin analysis results are known and are within the specifications. | For more details as regards sampling and analysis, see § 1.3 and §1.4.. In order to use this option, there must be an agreement between the producer and the customer. The customer must be informed of the analysis results, through means of an Analytical Report. |
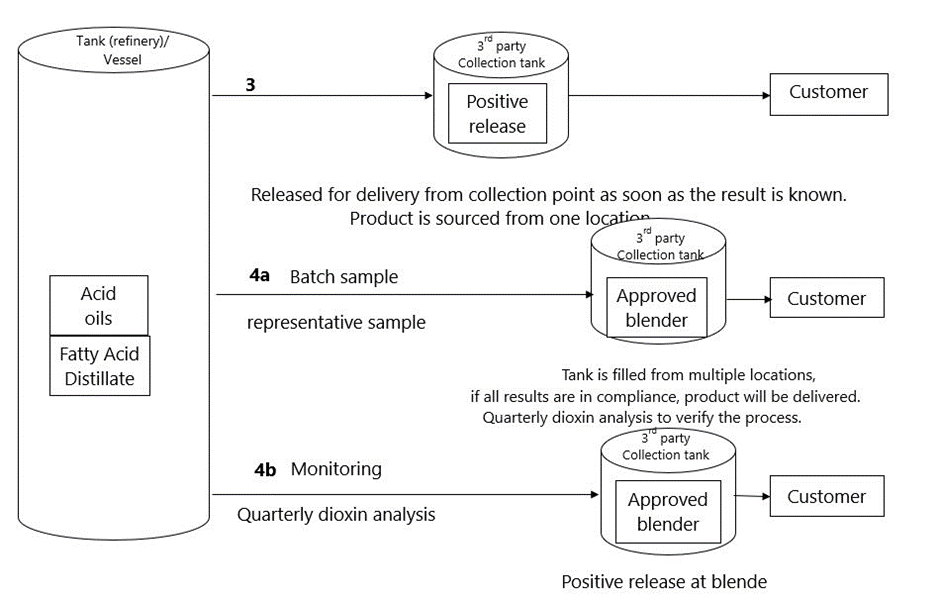
| 3 | The producer ships the product (from one plant) to a collection tank (located at another site). This can be a tank; located at his own facilities, or at a third-party thank. Sampling, will be performed in the collection tank. The collection tank is exclusively filled with one single batch. The tank can be loaded discontinuously, e.g. by truck, or by vessel, but the sum of the individual loads, loaded in the tank must correspond with the continuous production of a single plant. The product, will only be delivered from this tank to the customer, if the results of the dioxin and dioxin-like PCB’s analysis are known. | One single kind of fat/oil product. One producer/one production plant. Although the product is shipped from the production plant, the producer remains responsible for the required monitoring. He must arrange the proper corrective actions, if the analysis results exceed the product standards. The tank does not necessarily have to be located in the same country as the production site. The producer must have full control of the operational storage activities, or must have an agreement with the storage company, upon use of a third-party tank. Registration of production, transport and storage must be clear and show a complete balance. See for more details about sampling and analysis § 1.3 and §1.4. The customer must be informed of the analysis results, by means of an Analytical Report. |
| 4a | The producer ,must take a representative sample for the analysis of dioxin and dioxin-like PCB’s, before the products leave the production facility. The products are then shipped to a collection tank (which may be located at their own facilities, or with a third-party tank). When all samples, representing the contents of the tank, are falling within the required limits, as regards dioxin and dioxin-like PCB’s, the product may then be delivered, from the third-party collection tank, to the customers. For verification purposes, the producer will take a sample of the blend from the collection tank on a quarterly basis, for the analysis of dioxin and dioxin-like PCB’s. In case the contents of the tank, are not composed with batches, originating from one single production facility (option 3), the legal entity, operating the tank, will need to have an approval, as a fat blending operator. | This option, is only valid in case that product, delivered to the customer, is a feed material. When the product is a compound feed, this option 4a is not applicable. There may be more than one production plant involved, also from other producers. Although the product is shipped from the production plant, the producer stays responsible for the required monitoring. He must have arranged for proper corrective actions, in case the results of analysis exceed the product standards. The tank does not necessarily have to be located in the same country as the production site. The producer must have full control of the operational storage activities, or must have an agreement with the storage company, upon use of a third-party tank. The registration of production, transport and storage, must be clear, and must provide a complete balance. The file containing the analysis certificates must be complete, and must be clear. The customer must be informed of the analysis results, by means of all underlying analysis results, and the composition (including the proportion of the different components), unless the producer and customer agree, that the customer must be informed by means of a Conformity Note. The contents of the Conformity Note must be clear, unambiguous and verifiable. There must be a clear link between the Conformity Note, the delivered batch and the analysis certificates. The producer is responsible for the quarterly add-on monitoring. |
| 4b | Fat blending: different producers (which can be different plants and/or different legal entities), will deliver the product to the third-party collection tank. Sampling, will take place in the collection tank, at the fat blender’s facilities, after production of the fat blend. Each individual producer will monitor all products shipped to the third-party collection tank, via quarterly sampling (as an add-on to monitoring required). The individual producers are obliged to provide the monitoring results to the fat blender. | This option is mandatory, if the fat product is a compound feed. The product could be one single kind of fat/oil product, or a mixture of different fat/oil products. Product is owned by fat blender. The tank does not necessarily have to be located in same country as the production site. The producer must have full control of the operational storage activities, or must have an agreement with the storage company, upon use of a third-party tank. The fat blender is responsible for the quarterly add-on monitoring. The registration of production, transport and storage must be clear and provide a complete balance. The file, containing the analysis certificates must be complete and must be clear. The customer must be informed of the analysis results, by means of an Analytical Report of blend. |
*Example 1 to 4b: positive release not necessary in case the blend consists for 100% out of Acid Oils.
6. Monitoring protocol for By-products of the oils and fats industry
6.1. General requirements
6.1.1. Scope
Any product derived directly or indirectly from crude or recovered oils and fats by oleochemical or biodiesel processing or distillation, chemical or physical refining, other than:
- refined oils,
- products derived from refined oils,
- feed additives,
to be used in feed, from any origin.
The next products are out of scope:
- products, produced by an EU registered food operator
- crude or pure distilled fatty acids (13.6.6/13.6.7) derived from splitting of vegetable oil (2.20.1)
- products derived from fatty acids, covered under b
Note: See Appendix 1 for more details about products in scope of this protocol.
Helpful tip
‘To be used in feed’: it does not matter under which specification/status the product is purchased. If destination is feed, the relevant requirements in this document TS1.7 Monitoring apply.
6.1.2. Application
This protocol must be applied by GMP+ certified companies that:
- produce by-products (mentioned in 6.1.1) from the oil and fat industry.
- Trade / import by-products (mentioned in 6.1.1) from the oil and fat industry.
This protocol is not applicable for GMP+ certified companies that produce compound feed which is to be delivered to a livestock farmer.
6.2. Definitions
| Term | Explanation |
| MONG | Matter Organic Non-Glycerol See also F0.2 Definition list. |
6.3. Monitoring frequency
Batch by batch, 100% positive release. Batches/lots must be monitored before used in feed. Producer of the by-product is responsible unless agreed (in contract or another official document) to transfer this responsibility for monitoring to his customer. They must also agree that results are shared. Representative monitoring results need to accompany any delivered batch, also to customers.
6.4. Sampling
When shipped by sea vessel or barge
- Shipment to be carried out under a well-known, in the international trade accepted contract (FOSFA, NOFOTA, GROFOR) to assure
- Independent supervision
- Sampling per lot
- Safe previous cargoes and technical equipment
- When shipped by vehicles (tank/container):
- Sampling of each truck
6.5. Analysing
The below mentioned parameters must be analysed
- Fatty Acid profile
- Moisture and impurities
- Free Fatty Acid
- Dioxins, dioxin-like PCBs, non-Dioxin-like PCBs
- Pesticides
- Heavy metals (Arsenic, Cadmium, Mercury, Lead and Nickel)
- Mineral oil hydrocarbons (C10-C40)
- PAH’s
6.5.1. Information to the client
Information, which is generated as a result of application of this Appendix, must be unambiguous and must accompany every batch / shipment to demonstrate that requirements have been met.
Appendix 1
Product name and Number according to Reg. (EU) No 68/2013
| How to read |
| Reference is made to this appendix from both chapter 5 and chapter 6. It is good to keep the following in mind: Chapter 5: Chapter 5 gives the minimum frequency for analyzing dioxin and dioxin-like PCBs. For most oil and fat products the minimum frequency is stated in the tables in chapter 5. In this appendix 1 the classification for monitoring is only given for those products which are not classified in the tables in protocol 5, for companies which do not have a EU food registration (‘other’). Chapter 6: Chapter 6 requires (additional to chapter 5) for a few oil and fat product (from certain origin and from certain feedstocks) the minimum analysis frequency for a number of parameters. Oil and fat products within scope are indicated with ‘yes’ in the column ‘Within scope of § 6. |
| Number | Name | Description | § 5 Class for ‘other’ | In scope of § 6 | Remarks/examples of products falling under this number |
| 1.2.13 | Crude maize germ oil | Product obtained from maize germ | | No | |
| 1.6.13 | Rice bran oil | Oil extracted from stabilised rice bran | | No | |
| 2.20.1 | Vegetable oil and fat (2) | Oil and fat obtained from plants (excluding castor oil from the ricinus plant), it may be degummed, refined and/or hydrogenated. | | No | Palm oil stearin fraction; Rape seed stearin fraction; Sunflower stearin fraction |
| 2.20.2 | Used food factory vegetable oils | Vegetable oils having been used by food business operators in accordance with Regulation (EC) No 852/2004 for cooking purposes and which have not been in contact with meat, animal fats, fish or aquatic animals. | | No | |
| 2.21.1 | Crude lecithins | Product obtained during degumming of crude oil from oilseeds and oil fruits with water. Citric acid, phosphoric acid, sodium hydroxide or enzymes may be added during degumming of the crude oil. | | No | |
| 2.22.3 | Hemp oil | Oil obtained by pressing of hemp plants and seeds | | No | |
| 7.1.4 | Algal oil (1) | Oil obtained by extraction from algae. May contain up to 0,1 % antifoaming agents. | | No | |
| 9.2.1 | Animal fat | Product composed of fat from land animals, including invertebrates other than species pathogenic to humans and animals in all their life stages. If extracted with solvents, may contain up to 0,1 % hexane. | | No | |
| 10.4.6 | Fish oil | Oil obtained from fish or parts of fish followed by centrifugation to remove water (may include species specific details e.g. cod liver oil). | | No | |
| 10.4.7 | Fish oil, hydrogenated | Oil obtained from hydrogenation of fish oil | | No | |
| 13.6.1 | Acid oils from chemical refining (3) | Product obtained during the deacidification of oils and fats of vegetable or animal origin by means of alkali, followed by an acidulation with subsequent separation of the aqueous phase, containing free fatty acids, oils or fats and natural components of seeds, fruits tissues such as mono- and diglycerides, crude lecithin and fibres. | 2 | Yes | |
| 13.6.2 | Fatty acids esterified with glycerol (4)derived from 13.6.6 or 13.6.7, produced by splitting of vegetable oil (2.20.1) 14 | Glycerides obtained by esterification of fatty acids with glycerol. May contain up to 50 ppm Nickel from hydrogenation. | 4 | No | |
| Fatty acids esterified with glycerol(4), derived from 13.6.6 or 13.6.7, produced from other feedstock 14 | 2 | Yes | |||
| 13.6.3 | Mono di and tri glycerides of fatty acids(4), derived from 13.6.6 or 13.6.7, produced by splitting of vegetable oil (2.20.1)14 | Product consisting of mixtures of mono-, di- and triesters of glycerol with fatty acids. They may contain small amounts of free fatty acids and glycerol. May contain up to 50 ppm Nickel from hydrogenation. | 4 | No | |
| Mono di and tri glycerides of fatty acids(4), derived from 13.6.6 or 13.6.7, produced from other feedstock14 | 2 | Yes | |||
| 13.6.4 | Salts of fatty acids(4), derived from 13.6.6 or 13.6.7, produced by splitting of vegetable oil (2.20.1) 14 | Product obtained by reaction of fatty acids with at least four carbon atoms with calcium, magnesium, sodium or potassium hydroxides, oxides or salts. May contain up to 50 ppm Nickel from hydrogenation. | 4 | No | Analysis must be done on the fat component (e.g. PFAD) or on the end-product. |
| Salts of fatty acids(4), derived from 13.6.5, or salts of fatty acids(4), derived from 13.6.6 or 13.6.7, produced from other feedstock14 | 2 | Yes | |||
| 13.6.5 | Fatty acid distillates from physical refining (3) | Product obtained during the deacidification of oils and fats of vegetable or animal origin by means of distillation containing free fatty acids, oils or fats and natural components of seeds, fruits tissues such as mono- and diglycerides, sterols and tocopherols. | 2 | Yes | |
| 13.6.6 | Crude fatty acids from splitting(3) produced from vegetable oil (2.20.1)15 | Product obtained by oil/fat splitting. By definition it consists of crude fatty acids C6-C24, aliphatic, linear, monocarboxylic, saturated and unsaturated. May contain up to 50 ppm Nickel from hydrogenation. | 4 | No | Excluding crude fatty acids obtained by fermentation of organic matter, and enzymatic interesterification of oil. |
| Crude fatty acids from splitting(3) produced from other feedstock15 | 2 | Yes | | ||
| 13.6.7 | Pure distilled fatty acids from splitting(3), produced from vegetable oil (2.20.1)15 | Product obtained by the distillation of crude fatty acids from oil/fat splitting potentially plus hydrogenation. By definition it consists of pure distilled fatty acids C6-C24, phatic, linear, monocarboxylic, saturated and unsaturated. May contain up to 50 ppm Nickel from hydrogenation. | 4 | No | Excluding distilled fatty acids obtained by fermentation of organic matter, and enzymatic interesterification of oil. |
| Pure distilled fatty acids from splitting(3), produced from other feedstock15 | 2 | Yes | Ricin oleic acid (syn. Castor oil acid), CAS no.141-22-0, EC no. 205-470-2 Icosa-5,8,11,14-tetraenoic acid (syn. Arachidonic acid), CAS no. 506-32-1, EC no. 208-033-4; Hexanoic acid (syn. Caproic acid ) of vegetable origin, CAS no.142-62-1, EC no. 205-550-7; Octanoic acid (syn. Caprylic acid) of vegetable origin, CAS no.124-07-2, EC no. 204-677-5; Oleic acid (syn. octadec-9-enoic acid) of vegetable origin, CAS no. 112-80-1, EC no. 204-007-1; Linoleic acid (syn. 9,12-Octadecadienoic acid), CAS no. 60-33-3, EC no. 200-470-9; Linolenic acid (syn. (9Z,12Z,15Z)-9,12,15-Octadecatrienoic acid), CAS no. 463-40-1, EC no. 207-334-8; Stearic acid (syn. octadecanoic acid) of vegetable origin, CAS no. 57-11-4, EC no. 200-313-4 | ||
| 13.6.8 | Soap stocks (3) | Product obtained during the deacidification of vegetable oils and fats by means of aqueous calcium, magnesium, sodium or potassium hydroxide solution, containing salts of fatty acids, oils or fats and natural components of seeds, fruits or animal tissues such as mono- and diglycerides, crude lecithin and fibres. | | No | |
| 13.6.9 | Mono- and diglycerides of fatty acids esterified with organic acids(4) (5), derived from 13.6.6 or 13.6.7, produced by splitting of vegetable oil (2.20.1)14 | Mono- and diglycerides of fatty acids with at least four carbon atoms esterified with organic acids. | 4 | No | |
| Mono- and diglycerides of fatty acids esterified with organic acids(4) (5), derived from 13.6.6 or 13.6.7, produced from other feedstock14 | 2 | Yes | |||
| 13.6.10 | Sucrose esters of fatty acids(4), derived from 13.6.6 or 13.6.7, produced by splitting of vegetable oil (2.20.1) 14 | Esters of saccharose and fatty acids. | 4 | No | |
| Sucrose esters of fatty acids(4), derived from 13.6.6 or 13.6.7, produced from other feedstock14 | 2 | Yes | |||
| 13.6.11 | Sucroglycerides of fatty acids (4), derived from 13.6.6 or 13.6.7, produced by splitting of vegetable oil (2.20.1)14 | Mixture of esters of saccharose and mono and di-glycerides of fatty acids. | 4 | No | |
| Sucroglycerides of fatty acids(4) , derived from 13.6.6 or 13.6.7, produced from other feedstock 14 | 2 | Yes | |||
| 13.8.1 | Glycerine, crude [Glycerol, crude] | By-product obtained from:
May contain up to 0,5 % Methanol and up to 4 % of Matter Organic Non Glycerol (MONG) comprising of Fatty Acid Methyl Esters, Fatty Acid Ethyl Esters, Free Fatty Acids and Glycerides;
May contain up to 50 ppm Nickel from hydrogenation. | | No | |
| 13.8.2 | Glycerine [Glycerol] | Product obtained from:
May contain up to 50 ppm Nickel from hydrogenation. | | No | |
| 13.11.1 | Propylene glycol; [1,2- propanediol]; [propane- 1,2-diol] | Organic compound (a diol or double alcohol) with formula C3H8O2. It is a viscous liquid with a faintly sweet taste, hygroscopic and miscible with water, acetone, and chloroform. May contain up to 0,3 % di-propylene glycol. | | No | |
| 13.11.2 | Mono-esters of propy lene glycol and fatty acids (4) | Mono-esters of propylene glycol and fatty acids, alone or in mixtures with di-ester | 2 | Yes | |
| Explanation: 14 This product is out of scope of § 6 only in case produced/derived from fatty acids covered under 13.6.6 or 13.6.7, which are in their turn obtained by splitting of vegetable oil falling under the Catalogue of feed materials number 2.20.1. 15 The products 13.6.6 and 13.6.7 are out of scope of § 6 only in case they are produced by splitting. T he feedstock used to produce these products is vegetable oil falling under the Catalogue of feed materials number 2.20.1. When other products are used as the feedstock,, the products 13.6.6 and 13.6.7 are within scope of § 6. | |||||
| ( 1 ) The name must be supplemented by the species. ( 2 ) The name must be supplemented by the plant species. ( 3 ) The name must be supplemented by the indication of the botanical or animal origin. ( 4 ) The name must be amended or supplemented to specify the fatty acids used. ( 5 ) The name must be amended or supplemented to specify the organic acid. |
Risk Management tools
That was a lot of information to digest and one might ask, what is the next step? Luckily we can offer support for the GMP+ Community when doing this. We provide support by means of various tools and guidances but as each company has a shared responsibility to feed safety, and therefor tailor-made solutions cannot be offered. However, we do help by explaining requirements and provide background information about the requirements.
We have developed various supporting materials for the GMP+ Community. These include various tools, ranging from Frequently Asked Questions (FAQ) lists to webinars and events.
Supporting materials related to this document (Guidelines and FAQ’s)
We have made documents available which give guidance to the GMP+ requirements as laid down in the module GMP+ FSA and GMP+ FRA. These documents give examples, answers to frequently asked questions or background information.
GMP+ Monitoring database
The GMP+ Monitoring database contains analysis results from you and other users. It is possible to generate reports based on this data. We have a manual and a frequently asked questions document available.
Where to find more about the GMP+ International Risk Management tools? Fact sheets More information: GMP+ Platform Product list More information: Product List Risk Assessments More information: GMP+ Platform GMP+ Monitoring database More information: GMP+ Monitoring database Support documents More information: Support documents |



